Method for synthesizing lactam compound
A technology of artificial synthesis of lactam compounds, applied in the direction of fermentation, etc., can solve the problems of unstable structure, expensive metal catalysts, high reaction temperature, etc., and achieve high substrate specificity and high catalytic efficiency without being limited by light conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
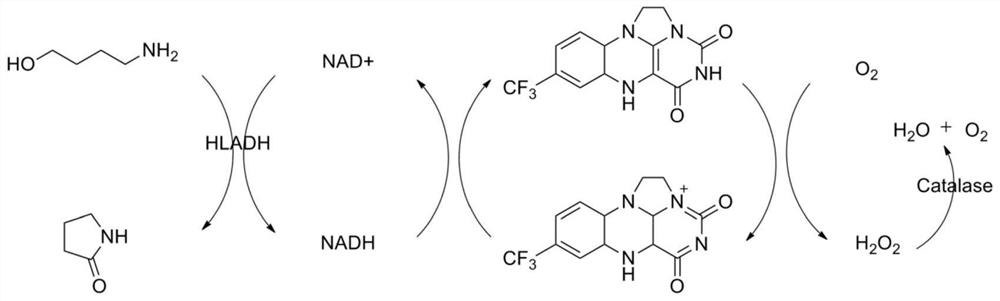
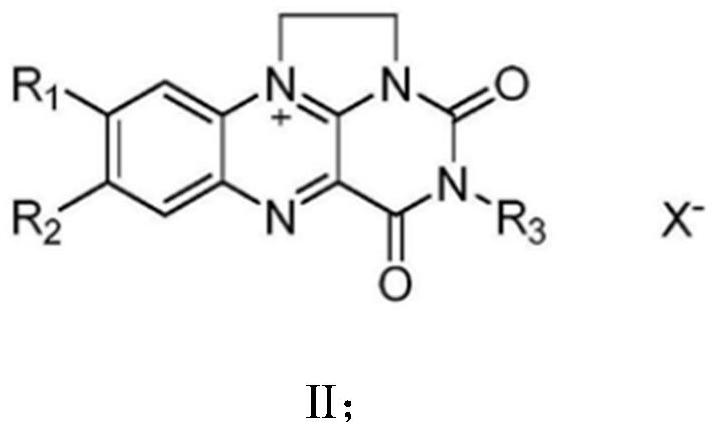
[0075] Regenerated NAD with 7-trifluoromethyl-N1,N10-vinylisoalloxazine chloride + Catalyst, coupled with horse liver alcohol dehydrogenase to catalyze 1-amino-4-butanol to 2-oxo-4-butyrolactam and H 2 o 2 , catalase further converts H 2 o 2 catalytic decomposition to H 2 O and O 2 .
[0076] Its reaction schematic diagram is as follows figure 1 As shown, 1-amino-4-butanol was catalyzed by horse liver alcohol dehydrogenase, 7-trifluoromethyl-N1,N10-vinylisoxazine chloride and NAD + Under the regeneration reaction system constituted, the lactamization product 2-oxo-4-butyrolactam and H 2 o 2 , catalase further converts H 2 o 2 catalytic decomposition to H 2 O and O 2 .
[0077] In 10 mL of 50 mM pH 8 potassium phosphate buffer, 1-amino-4-butanol 20 mM, NAD + 1mM, 7-trifluoromethyl-N1, N10-vinylisoalloxazine chloride 0.5mM, horse liver alcohol dehydrogenase 5U / mL, catalase 20U / mL, the reaction solution is connected with the outside air, in 30° C., 200 rpm shaker, ...
Embodiment 2
[0080] 7-Trifluoromethyl-N1,N10-vinylisoalloxazine chloride as regenerated NAD + Catalyst, coupled with horse liver alcohol dehydrogenase to catalyze 4-amino-2-methyl-1-butanol to (R)-3-methylpyrrolidone-2-one and H 2 o 2 , catalase further converts H 2 o 2 catalytic decomposition to H 2 O and O 2 .
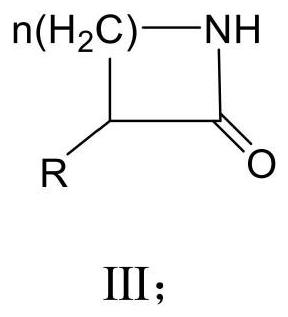
[0081] In 10 mL of 50 mM pH 8 potassium phosphate buffer, 4-amino-2-methyl-1-butanol 20 mM, NAD + 1mM, 7-trifluoromethyl-N1, N10-vinylisoalloxazine chloride 0.5mM, horse liver alcohol dehydrogenase 5U / mL, catalase 20U / mL, the reaction solution was communicated with the outside air, in 30° C., 200 rpm shaker, reacted for 24 hours, and by gas phase quantitative analysis, the yield of (R)-3-methylpyrrolidone-2-one represented by formula III-II was 95%.
[0082]
Embodiment 3
[0084] 7-Trifluoromethyl-N1,N10-vinylisoalloxazine chloride as regenerated NAD + Catalyst, coupled with horse liver alcohol dehydrogenase to catalyze 1-amino-5-hydroxypentane to 5-aminovaleric acid lactam and H 2 o 2 , catalase further converts H 2 o 2 catalytic decomposition to H 2 O and O 2 .
[0085] In 10 mL of 50 mM pH 8 potassium phosphate buffer, 1-amino-5-hydroxypentane 20 mM, NAD + 1mM, 7-trifluoromethyl-N1, N10-vinylisoalloxazine chloride 0.5mM, horse liver alcohol dehydrogenase 5U / mL, catalase 20U / mL, the reaction solution was communicated with the outside air, in 30° C., 200 rpm shaker, reacted for 12 hours, and by gas phase quantitative analysis, the yield of 5-aminovalerolactam represented by formula III-III was 35%.
[0086]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com