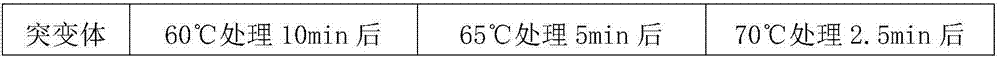Glucose oxidase mutant
A technology of glucose oxidase and mutants, applied in the directions of oxidoreductase, enzymes, biochemical equipment and methods, etc., can solve problems such as the inability to guarantee the stability of enzyme preparations, the uniformity of enzyme preparation distribution, application limitations, and increased equipment investment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Obtainment of glucose oxidase heat-resistant single point mutant
[0048] 1.1 Amplification of the glucose oxidase gene
[0049] The Aspergillus niger genome was used as a template for PCR amplification, and the PCR primers GOD-F1 and GOD-R1 were as follows:
[0050] GOD-F1: GGTATTGAGGCATCTTTGTTGAC
[0051] GOD-R1: TTATTGCATAGAAGCGTAATC
[0052] The PCR product was recovered by gel, connected to the pEASY-T vector, transformed into Escherichia coli DH5α, and the correct transformant was picked for sequencing. Sequencing results showed that the nucleotide sequence of the amplified gene fragment was SEQ ID NO: 2, and the encoded amino acid sequence was SEQ ID NO: 1. Through NCBI BLAST comparison, it was found that the sequence similarity between SEQ ID NO: 1 and the glucose oxidase from Aspergillus niger was as high as 100%, so it was determined that the gene obtained by PCR was the glucose oxidase gene and named GOD.
[0053] 1.2 Amplification and synthesis...
Embodiment 2
[0096] Example 2 Saturation Mutation Screening of Glucose Oxidase Thermostable Single Point Mutants
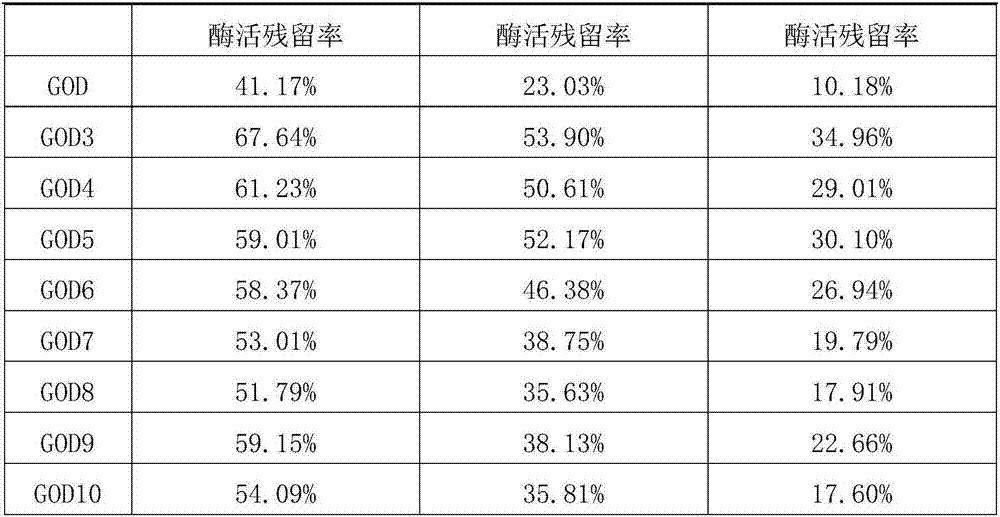
[0097] Using the method for screening and obtaining heat-resistant mutants described in Example 1, the 64th and 415th amino acids of glucose oxidase GOD were subjected to saturation site mutations. Further, thermostability experiments were carried out on the obtained mutants respectively.
[0098] The results show that when the 64th amino acid of glucose oxidase GOD is mutated from A to L, or K, or M, or F, or N, or Q, they all show better heat resistance than the wild type. The mutants are respectively named GOD3, GOD4, GOD5, GOD6, GOD7, GOD8, and their coding nucleotide sequences are respectively SEQ ID NO: 5, SEQ ID NO: 6, SEQ ID NO: 7, SEQ ID NO: 8, SEQ ID NO: 9, SEQ ID NO: 10;
[0099] When the 415th amino acid of glucose oxidase GOD is mutated from A to R or N, they all show better heat resistance than the wild type, and the above mutants are named GOD9 and GOD10 respe...
Embodiment 3
[0104] Example 3 Obtainment of Glucose Oxidase Thermostable Two-Point Combination Mutant
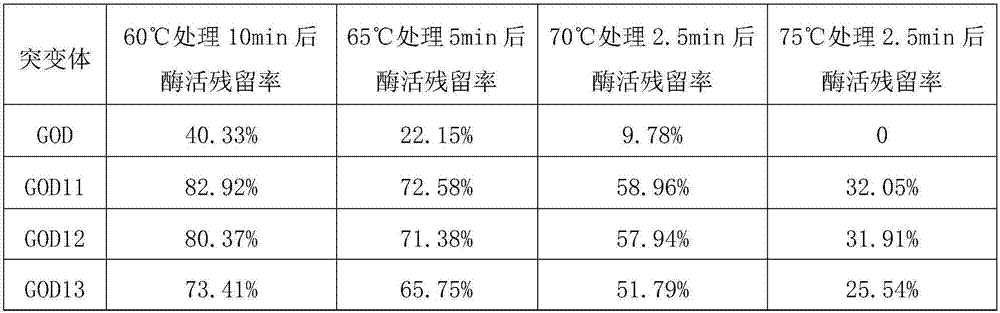
[0105] Using the method for screening and obtaining mutants described in Example 1, the mutation combination screening was performed on the 64th and 415th heat-resistant sites of glucose oxidase GOD. Further, thermostability experiments were carried out on the obtained mutants respectively.
[0106] The results show that the two point mutation combinations with better heat resistance than the above single point mutants include: A64R+A415K, or A64R+A415R, or A64R+A415N, or A64L+A415K, or A64L+A415R, or A64L+A415N, Or A64K+A415K, or A64K+A415R, or A64K+A415N, or A64M+A415K, or A64M+A415R, or A64M+A415N, or A64F+A415K, or A64F+A415R, or A64F+A415N, or A64N+A415K, Or A64N+A415R, or A64N+A415N, or A64Q+A415K, or A64Q+A415R, or A64Q+A415N.
[0107] The residual rate of glucose oxidase activity of the two-point mutant is generally increased by 30% to 235% compared with the above-mentioned sin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com