Recombinant PAI-1 inhibitor, composition containing recombinant PAI-1 inhibitor, and uses of recombinant PAI-1 inhibitor and composition in treatment and detection
A PAI-1, inhibitor technology, applied in the field of biomedicine, can solve the problems of easily induced thrombosis, inhibited local fibrinolytic activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
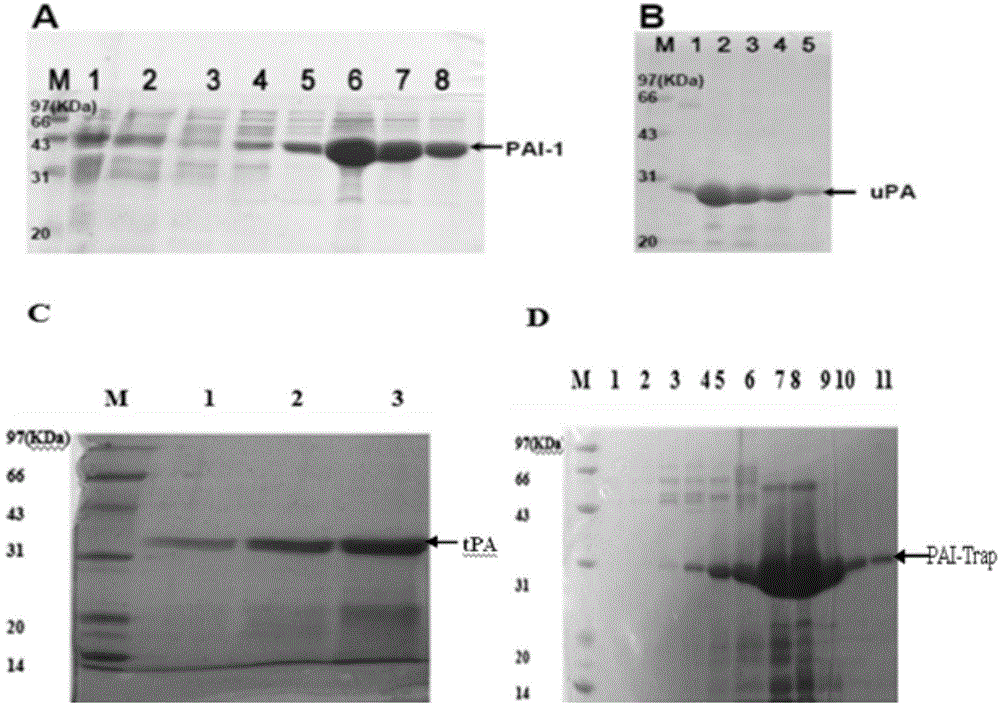
[0102] Cloning of embodiment one PAI-Trap gene, and expression and purification of PAI-Trap protein
[0103] (1) The PAI-Trap here is constructed by carrying out the S195A mutation of the uPA hydrolase domain (uPA-SPD, whose nucleotide sequence is SEQ ID NO.10), also referred to as uPA-S195A(I16-E244) or uPA-S195A (amino acids named as chymotrypsinogen number):
[0104] The uPA-SPD-pPicZαA plasmid (Invitrogen) was used as a template.
[0105] Primer design:
[0106] Sense primer: 5'-GCCAGGGAGACTCAGGGGGACC-3' (SEQ ID NO.6)
[0107] Antisense: 5'-GGTCCCCCCTGAGTCTCCCCTG-3' (SEQ ID NO.7)
[0108] PCR system:
[0109] wxya 2 O 32ul
[0110] 5HF buffer (Thermal scientific) 10ul
[0111] 2mM dNTP (Shanghai Sangong) 5ul
[0112] 20mM sense primer 1ul
[0113] 20mM antisense primer 1ul
[0114] Template (20ng)0.5ul
[0115] Phuison (Thermal scientific) 0.5ul
[0116] PCR conditions:
[0117] 98°C for 3 minutes;
[0118] 25 loops:
[0119] 98°C for 20 seconds,
[0120] 6...
Embodiment 2
[0166] Expression and purification of embodiment two PAI-1, uPA and tPA
[0167] (1) Expression and purification of PAI-1: The recombinant PAI-1 expression plasmid pT7-PL (a gift from Professor Zhou Aiwu of Shanghai Jiao Tong University) was transformed into BL21 Escherichia coli strain (Invitrogen). The recombinant expression strain was inoculated in LB (1 tryptone, 0.5% yeast extract powder, 1% NaCl, containing 100mg / LAmp) and cultured overnight at 37°C, then inoculated in fresh LB (1 tryptone, 0.5% yeast extract powder) at a ratio of 1:100 , 1% NaCl, containing 100mg / L Amp) at 37°C to OD600 of about 0.6, induced with 0.5mM IPTG at 20°C for 6 hours, centrifuged at 10,000rpm for 10 minutes, collected the bacteria, and washed with buffer A (25mM MESpH6.1 , 1M NaCl), ultrasonically crushed, and centrifuged at 10,000rpm for 30 minutes, the supernatant was combined with Ni-NTA (Qiagen) column for 2 hours, and gradient elution was performed with imidazole-containing buffer A, and ...
Embodiment 3
[0173] The activity measurement of embodiment three PAI-Trap
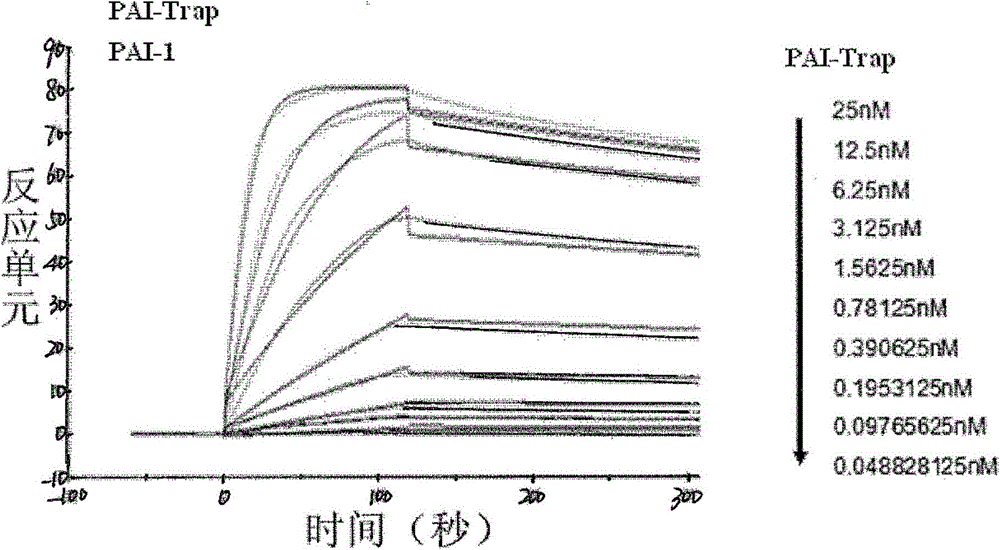
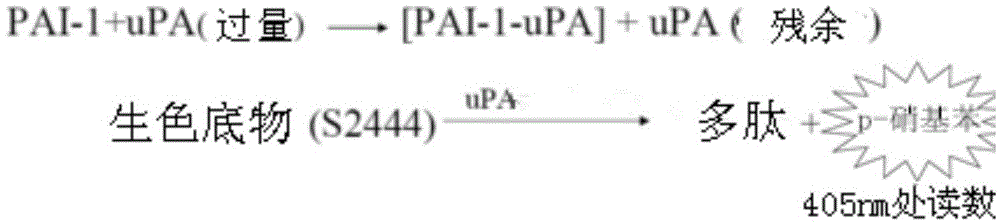
[0174] The enzyme activity test of PAI-1 was mainly carried out according to the reported Chromogenic Assay (Liang, A., et al., 2005). Briefly, in a 100 μL system (50mM Tris pH7.4, 150mM NaCl), different concentrations of PAI-Trap were pre-incubated with 5nM PAI-1 (final concentration) for 10 minutes, then 5nM uPA was added to mix and react at room temperature After 10 minutes, the luminescent substrate S2444 (Chromogenix) was added and immediately placed in a BioTek Synergy4 microplate reader at 405 nm, 15 seconds / reading for 10 minutes. Each test was repeated at least 3 times. IC of PAI-trap to PAI-1 50 The nonlinear regression (Sigmoidal) in Origin7.5 software was used for fitting.
[0175] The competitive inhibition of PAI-1 by a typical PAI-trapSEQ ID NO.4 is as follows image 3 shown. Inhibitory IC of PAI-trap on PAI-1 50 The results are shown in Table 1. These PAI-Traps all have strong inhibitory abili...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com