Variant of Kex2 enzyme and stable expression method
A stable and variant technology, applied in the biological field, can solve the problems of cumbersome protein expression induction process, low expression level, unfavorable industrial production, etc., and achieve the effect of retaining biological activity, high enzyme activity, and convenient purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Embodiment 1. Kex2 protease truncation body or variant design
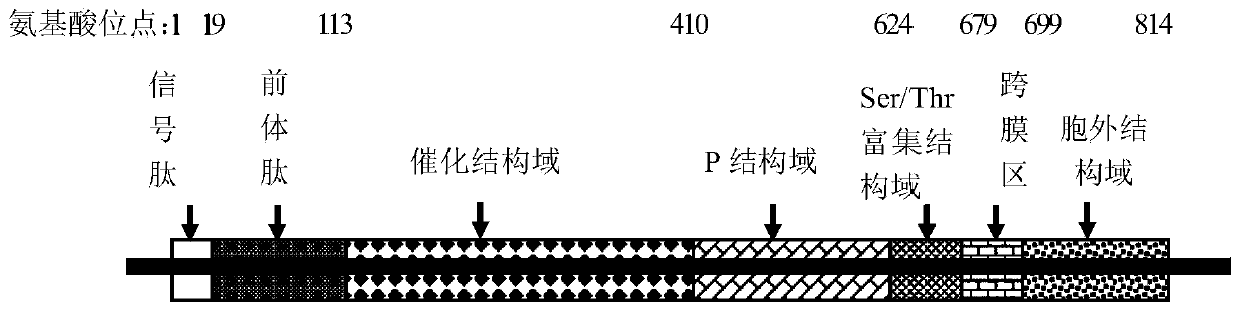
[0078] Kex2 consists of 814 amino acid residues (SEQ ID NO.1), the whole molecule contains signal peptide (1-19aa), precursor peptide (20-113aa residues), catalytic domain (114-410aa residues), P Domain (411-624aa residues), Ser / Thr rich domain (625-679aa residues), transmembrane region (680-699aa residues) and extracellular domain (700-814aa residues), kex2 structure Such as figure 1 shown.
[0079] The 1-613th truncated body of Kex2, based on the amino acid sequence of the full-length kex2 enzyme, wherein the 225th, 436th, and 503rd positions are mutated from K to A or P, and at the same time, the C-terminal of the truncated body is added or not Add the His tag.
[0080] The 1-667th truncated body of Kex2, based on the amino acid sequence of the full-length kex2 enzyme, wherein the 225th, 436th, and 503rd positions are mutated from K to A or P, and at the same time, the C-terminus of the truncated body...
Embodiment 2
[0087] Example 2. Construction of recombinant Pichia pastoris
[0088] The recombinant expression plasmid containing kex2 truncation or variant is linearized by Avr ll or BspH I enzyme, and then the linearized recombinant expression plasmid is recovered by using TaKaRa MiniBEST DNA Fragment Purification Kit Ver.4.0.
[0089]The constructed linearized recombinant expression plasmid is transferred into Pichia pastoris X33 or gs115 competent cells by electroporation, so as to obtain recombinant Pichia pastoris.
[0090] The pGAPZa A expression plasmid contains the Zeocin antibiotic selection gene, and the recombinant Pichia pastoris that stably and efficiently secretes and expresses kex2 can be screened by using different concentrations of Zeocin antibiotics.
[0091] A YPD plate (1% Yeast Extraction, 2% Peptone, 2% glucose) with a Zeocin concentration of 100 μg / ml was prepared for screening recombinant Pichia pastoris with stable and high-efficiency secretory expression.
Embodiment 3
[0092] Example 3. High-efficiency secretory expression of Kex2 protease
[0093] From the YPD plate containing Zeocin antibiotics, randomly pick single clones that grow well, inoculate them into 3ml of YPD medium, set the culture conditions at 28-30°C, 250-300rpm, cultivate for about 24h, and then transfer to 0.2% volume Connect to fresh YPD medium, continue to cultivate, and take samples at about 24h, 48h, and 72h of culture to identify protein expression results, such as Figure 2-4 As shown (A: indicates the protein expression results in host X33, 1-18 in the figure respectively indicate the protein expression results of 1-18 clones picked; B: indicates the protein expression results in host gs115, in the figure 1-18 18 represent the protein expression results of 1-18 selected clones, respectively).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com