A kind of glutamine synthetase mutant and its application
A technology of glutamine and synthetase, which is applied in the field of biomedicine, can solve the problems of weakened enzyme activity, affecting the growth state of CHO stable cell lines, and CHO cell toxicity, and achieves the goals of reducing usage, good application value, and enhancing affinity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1 Structural prediction and mutant prediction of CHO glutamine synthetase
[0085] 1. Structural prediction of CHO glutamine synthetase
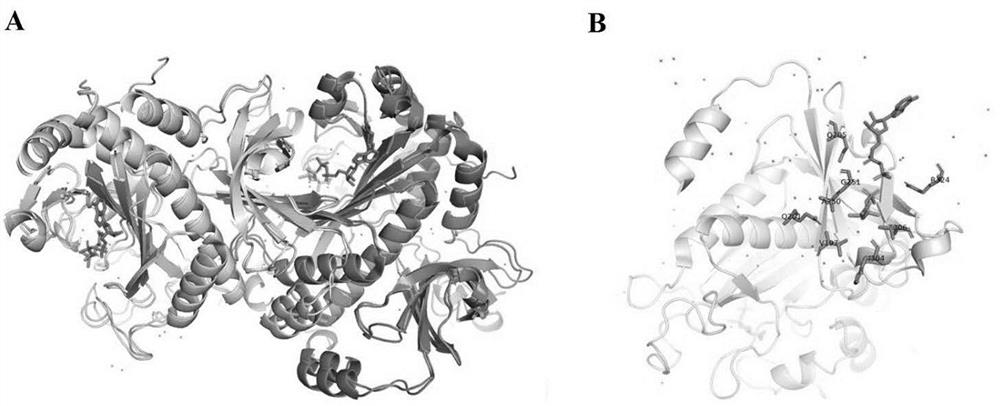
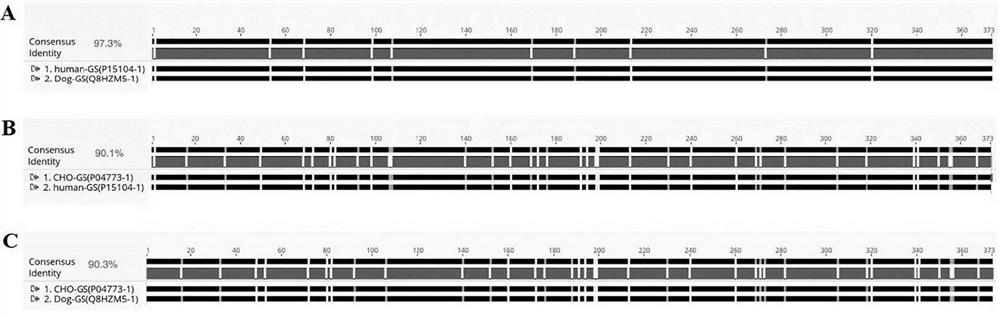
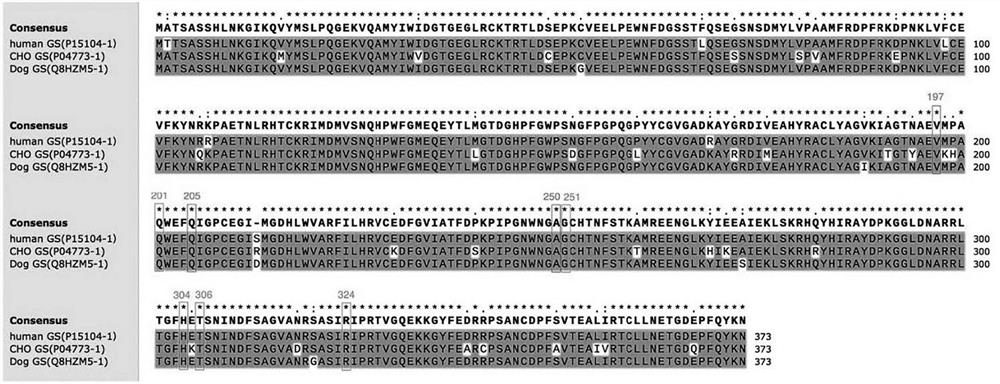
[0086] Although there is no information on the crystal structure of CHO glutamine synthetase, Krajewski et al. published the crystal structure of human and dog glutamine synthetase 2QC8 (Crystal structure of human glutamine synthetase in complex with ADP and methionine sulfoximine phosphate in 2008) )、2QJW(Crystal structure of human glutamine synthetase in complex with ADP andphosphate)和2UU7(Crystal structure of apo glutamine synthetase from dog)(参考文献为Crystal structures of mammalian glutamine synthetases illustratesubstrate-induced conformational changes and provide opportunities for drugand herbicide design . Krajewski, W.W., Collins, R., Holmberg-Schiavone, L., Jones, T.A., Karlberg, T., Mowbray, S.L. (2008) J Mol Biol 375: 217-228). Amino acid sequence alignment results show that Chinese hamster (Cricetulus griseus; CHO) g...
Embodiment 2
[0095] Example 2 Determination of Glutamine Synthetase Activity
[0096] 1. Construction of firefly luciferase-GS co-expression vector
[0097] The GS mutant genes (GSm1, GSm9-12), wild-type GS gene (GSwt) and R324C mutant corresponding to CHO predicted above were cloned into the eukaryotic expression vector pcDNA3.1 ( +) In the pcDNA3.1-LUCI-Furin T2A vector with modified backbone (see Figure 4 ). The optimized self-cleaving peptide structure of Furin T2A has an amino acid sequence of RRKRGSGEGRGSLLTCGDVEENPGP (SEQ ID NO: 15), and its cleavage efficiency is close to 100%, which can basically guarantee the equal expression of firefly luciferase and GS to be tested, so it can The expression of GS in each experimental group was corrected by quantifying firefly luciferase.
[0098] 2. GS activity assay method
[0099] Use a six-well plate to adhere to culture CHOK1SV GS-KO cells (glutamine synthetase deficiency) (LONZA cells), add 2 mL to each well at a density of 10 5 cel...
Embodiment 3
[0104] Example 3 Construction of antibody eukaryotic expression vector
[0105] Backbone vector pDCH2.1 for testing antibody expression (see Figure 6) is spliced by whole gene synthesis after design, and its components include: EF-1α promoter, CMV enhancer, CMV promoter, WPRE element, HSV TK terminator, f1 ori replication initiation site, SV40 promoter, wild Type CHO GS selection marker, SV40 terminator, ori replication origin site, prokaryotic ampicillin resistance selection marker and AmpR promoter, etc. The vector skeleton is suitable for monoclonal antibody double-gene expression, in which the light chain cloning site is HindIII-BsiWI-BamHI, and the heavy chain cloning site is ClaI-NheI-XhoI. The wild-type CHO GS screening marker can be replaced by the low-activity GS mutant screened above by the NotI-EcoRI double enzyme digestion method. The constructed and prepared plasmid can be used for stable transfection screening of CHO K1 after PvuI linearization and recovery....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com