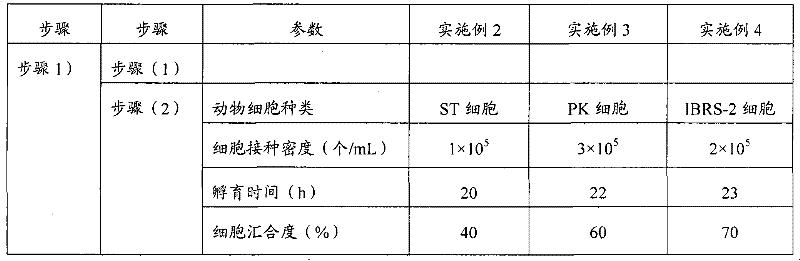
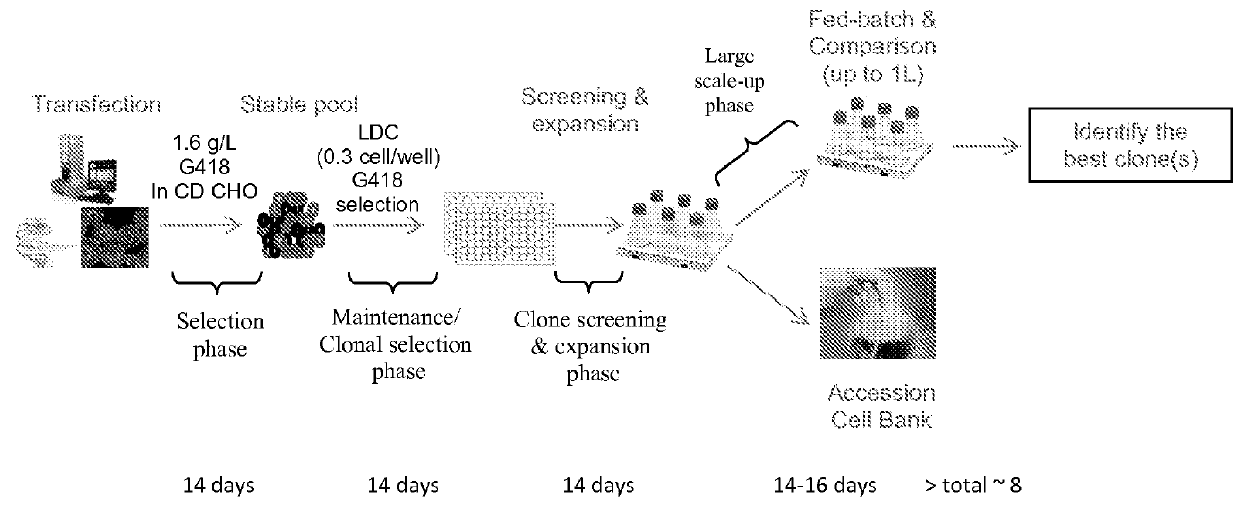
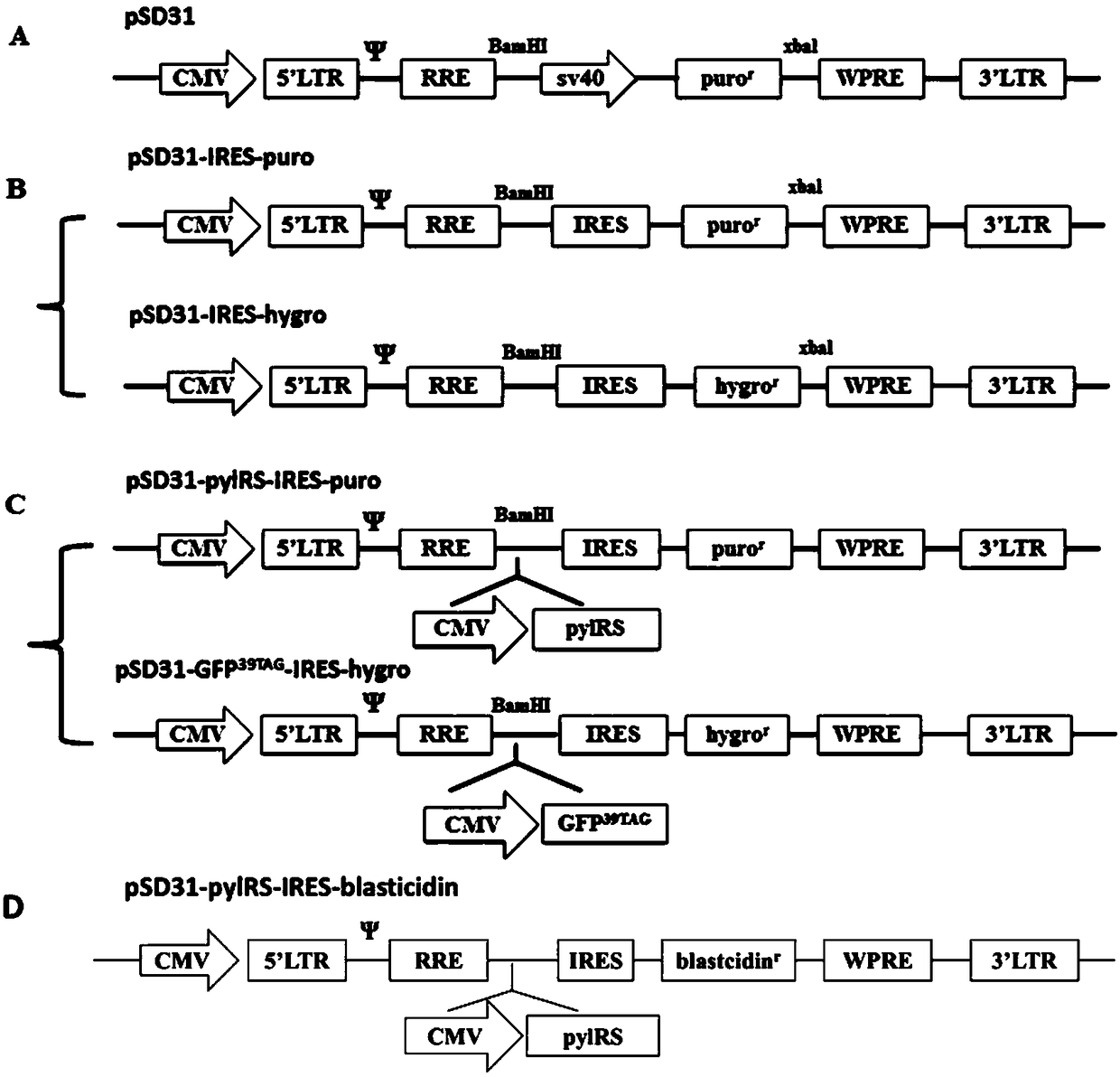
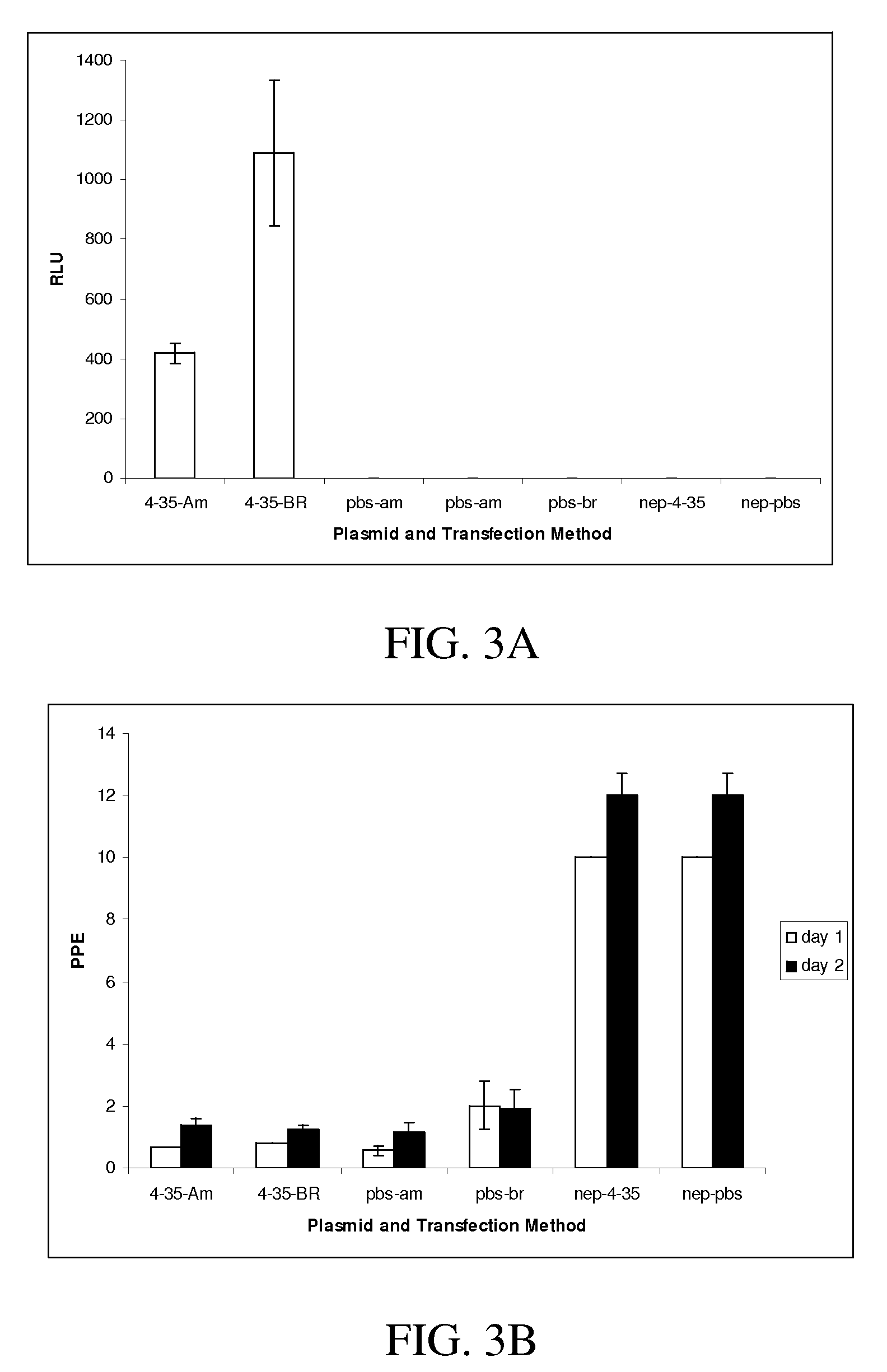
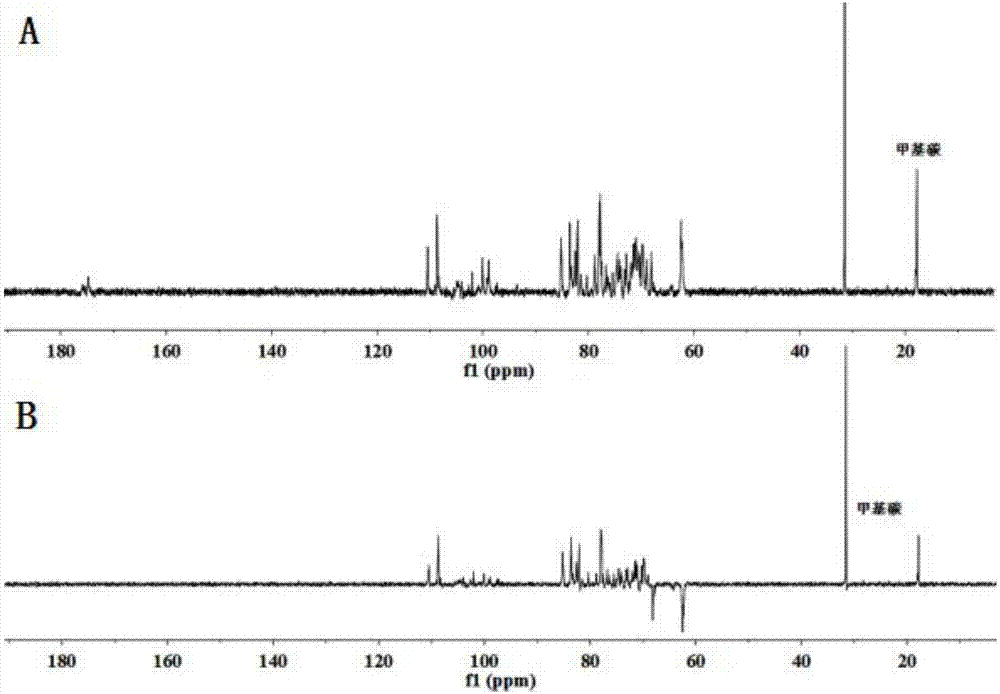
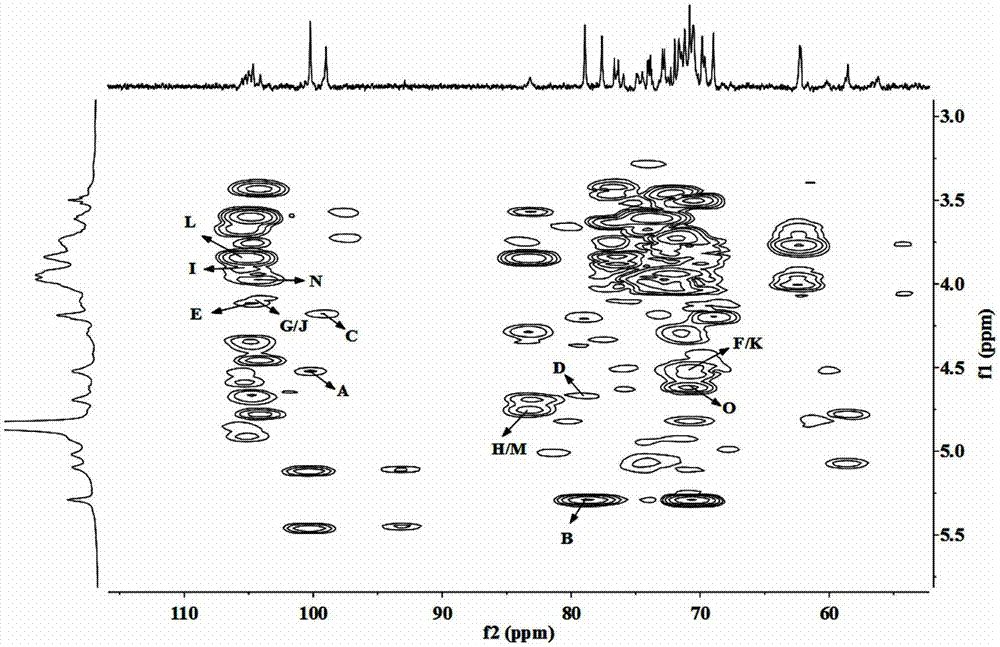
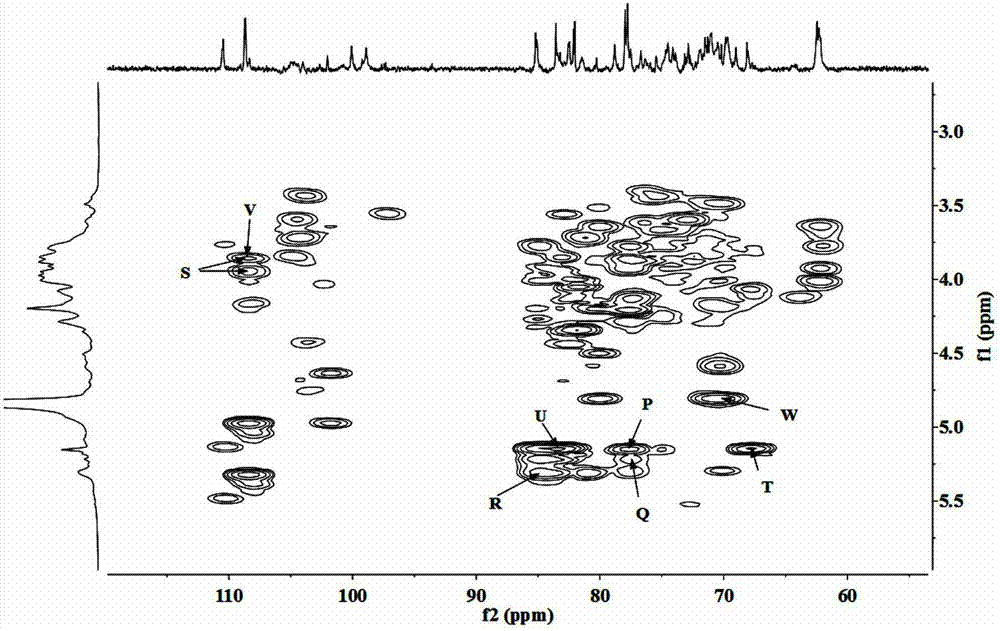
Patents
Literature
139 results about "Stable transfection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Stable transfection: A form of transfection experiment designed to produce permanent lines of cultured cells with a new gene inserted into their genome.
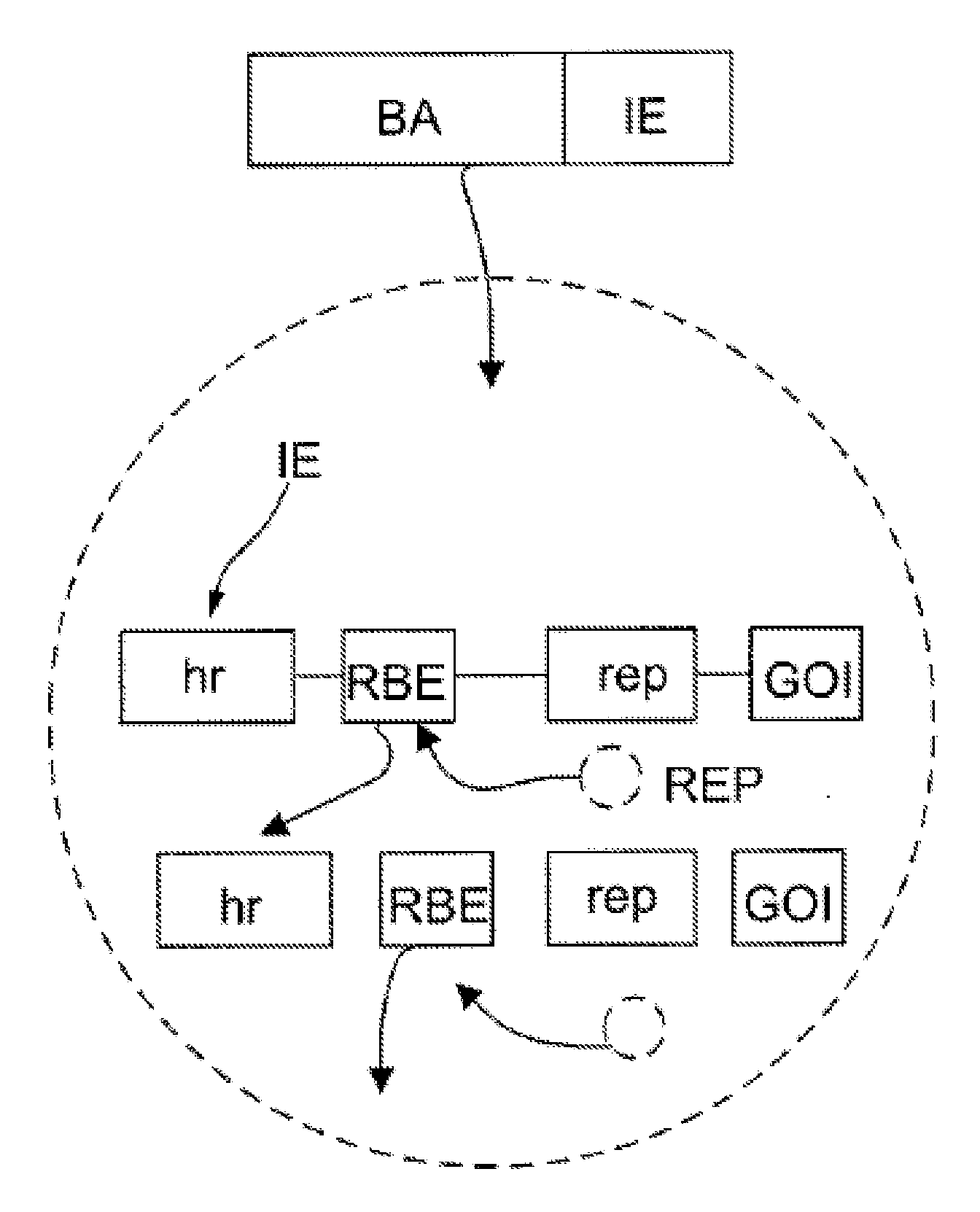
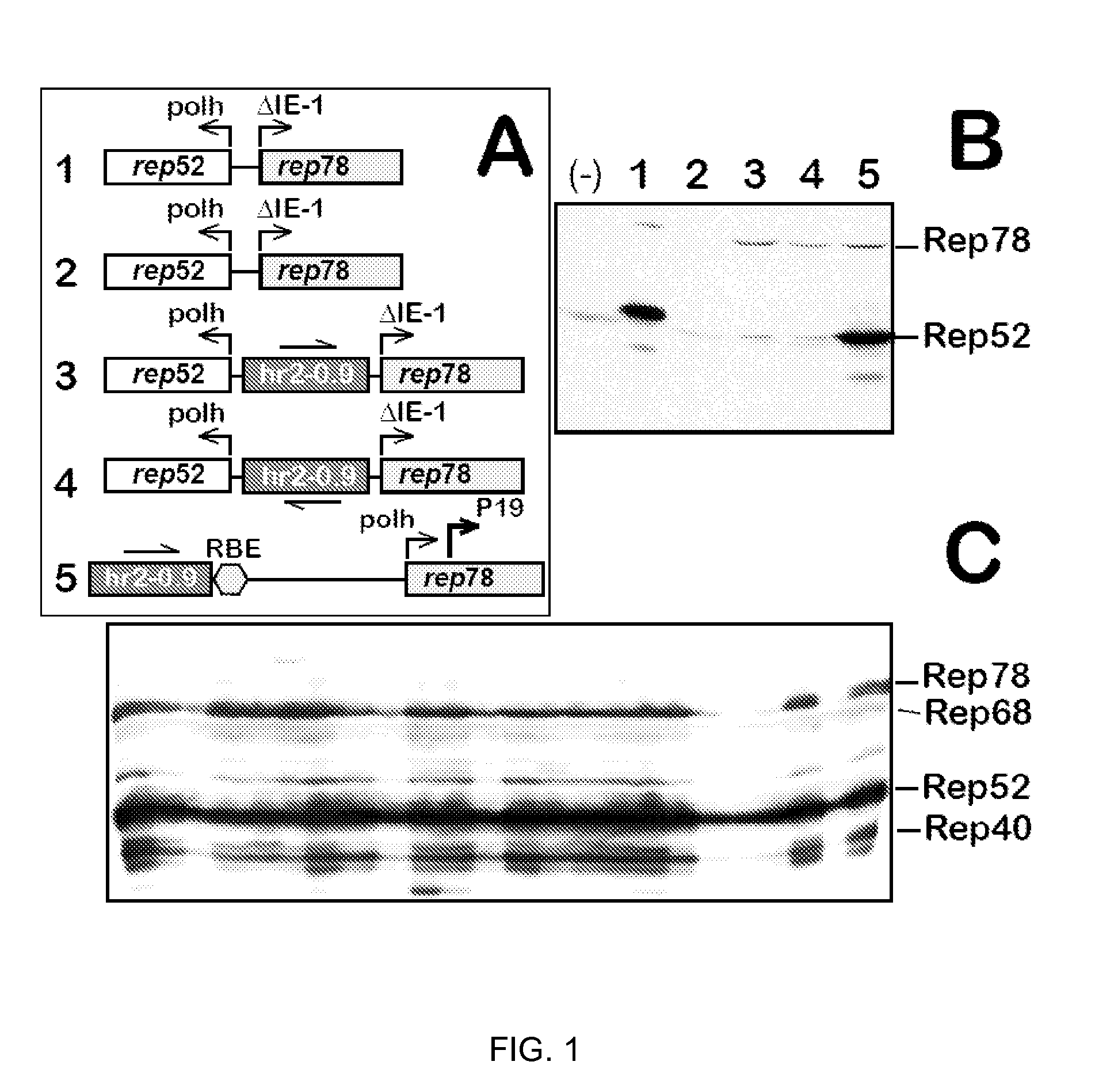
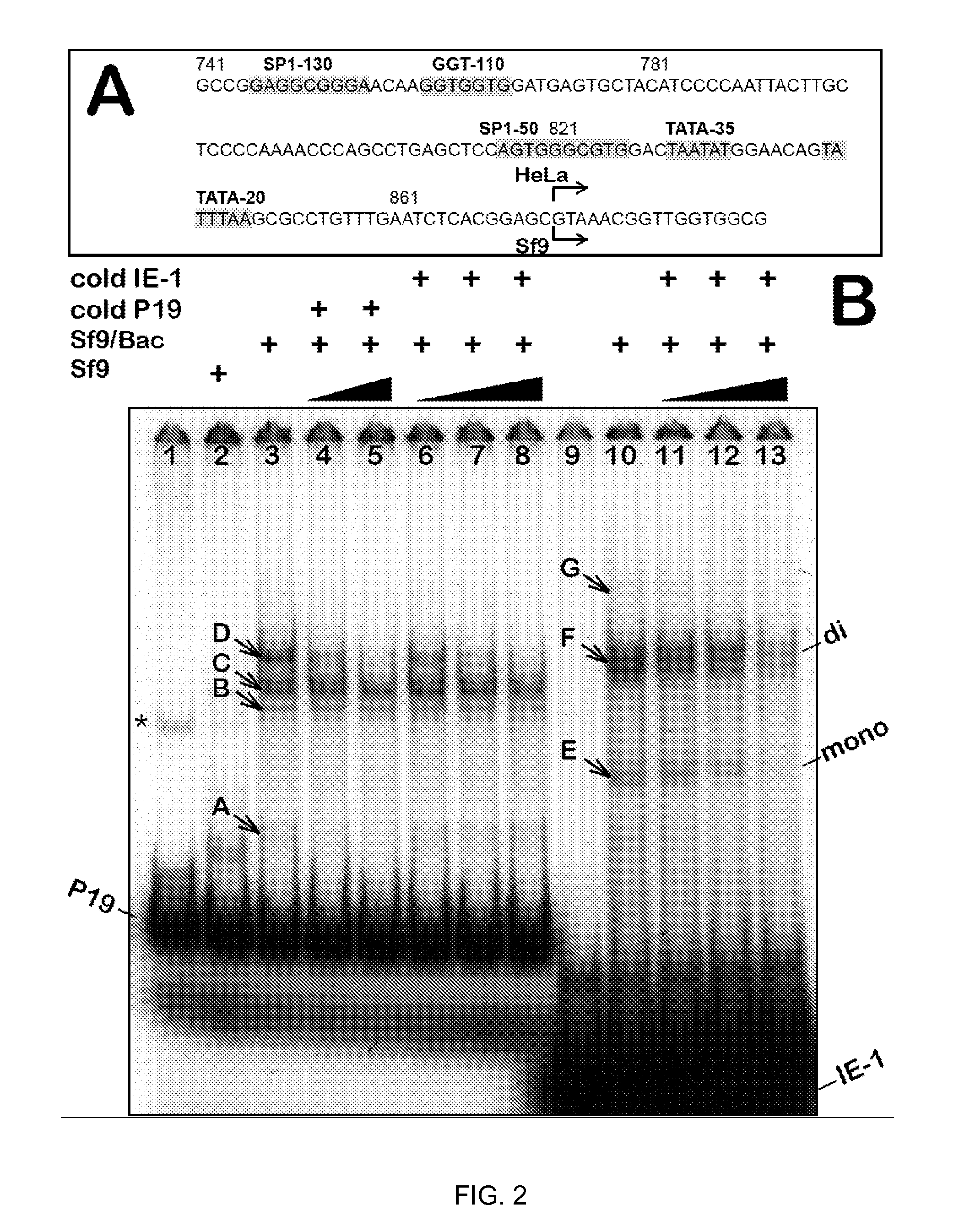
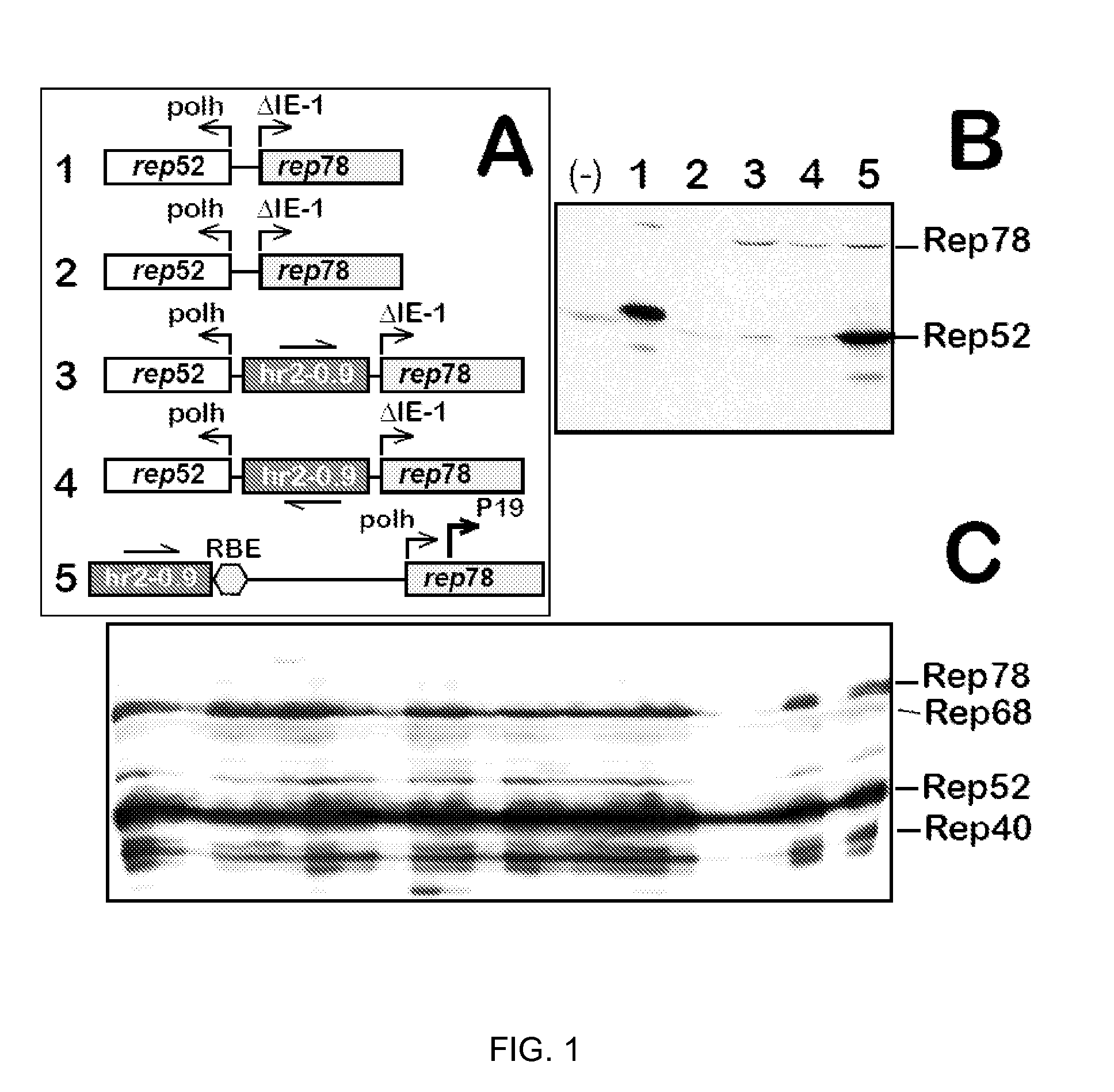
Inducible System for Highly Efficient Production of Recombinant Adeno-Associated Virus (rAAV) Vectors
ActiveUS20120100606A1High production costHigh yieldAnimal cellsNucleic acid vectorDiseaseClinical grade
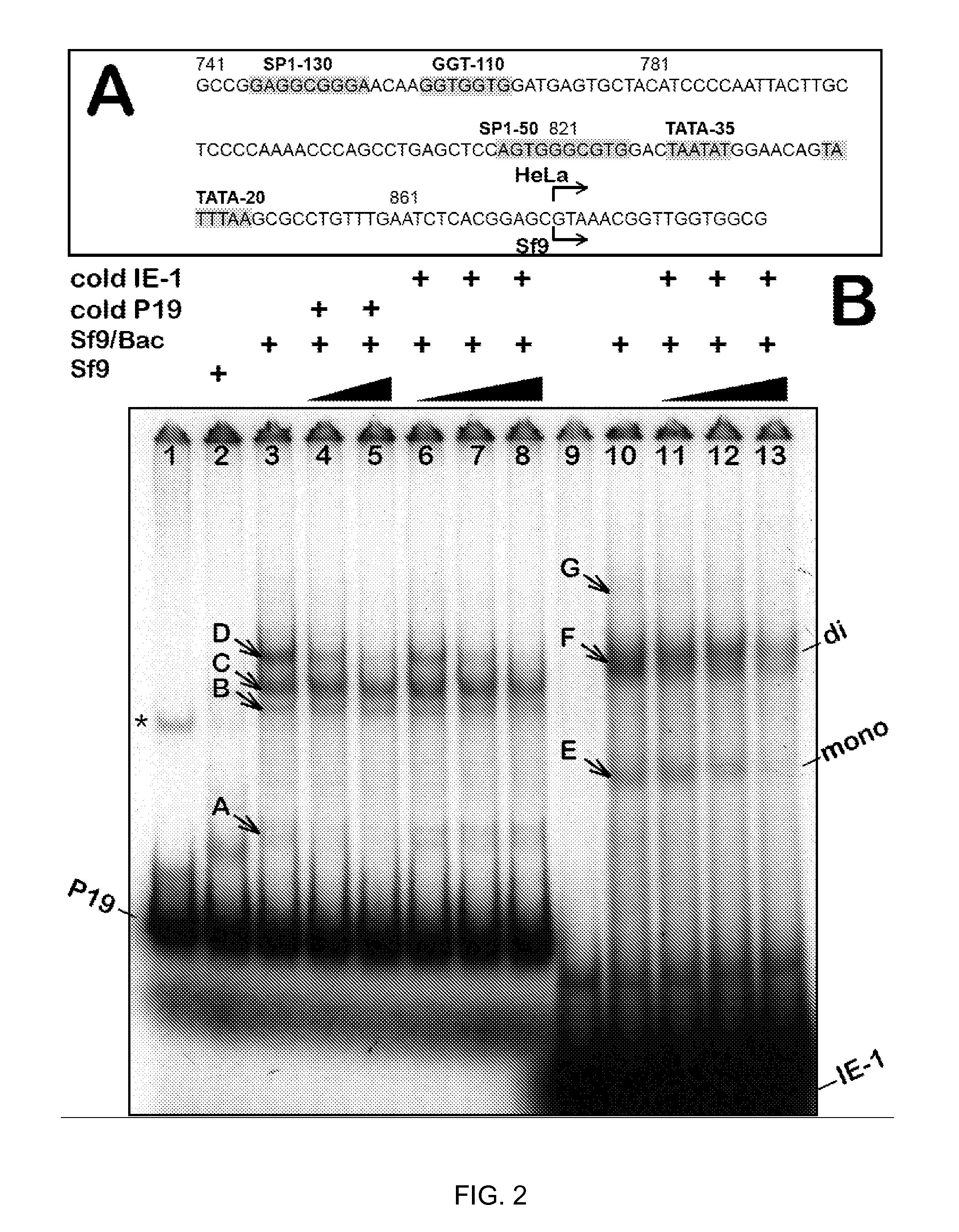
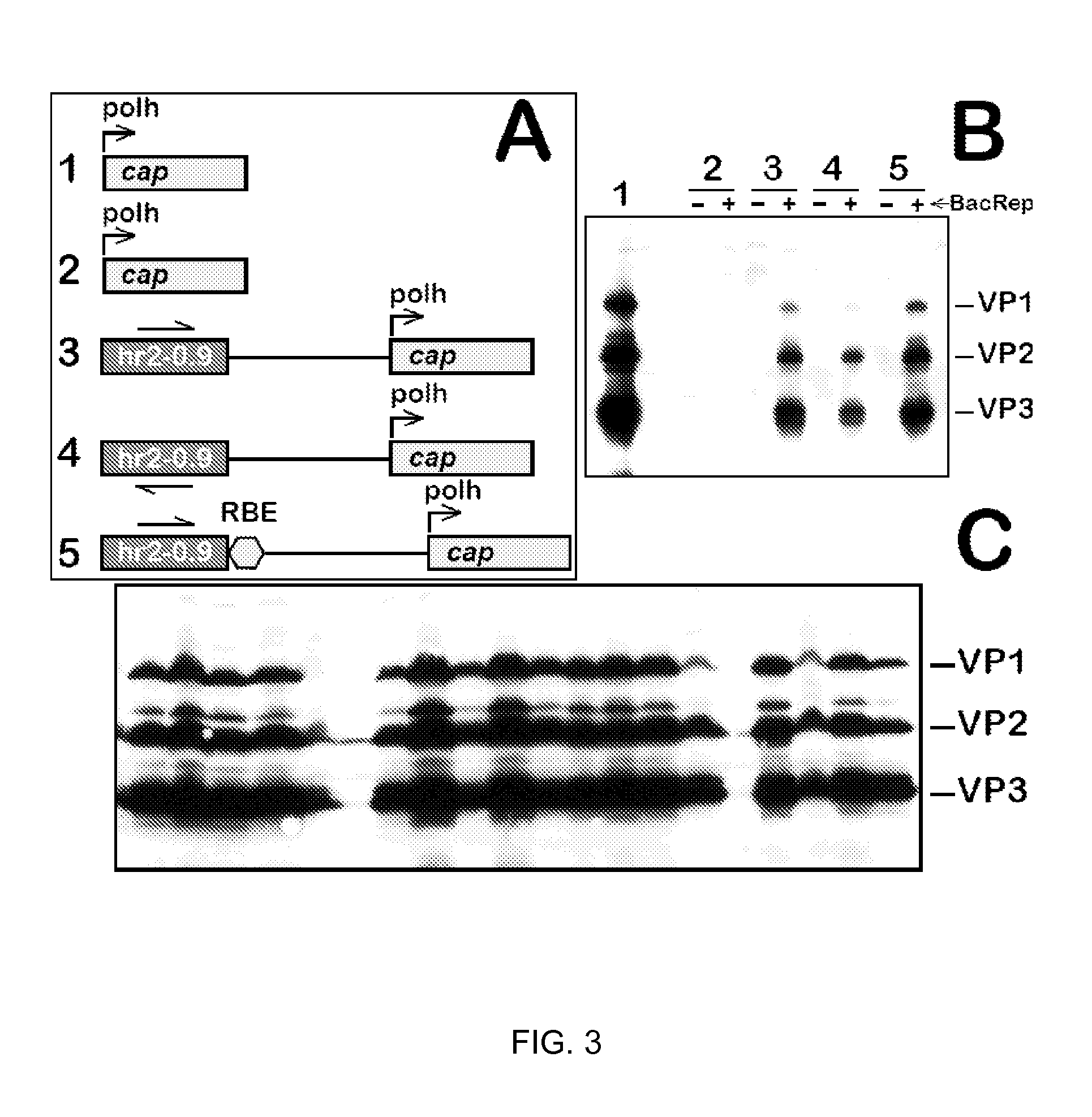
Production of clinical grade gene therapy vectors for human trials remains a major hurdle in advancing cures for a number of otherwise incurable diseases. Disclosed herein are systems based on a stably trans formed insect cell lines harboring helper genes required for vector production. Specifically exemplified are system embodiments that take advantage of DNA regulatory elements from two unrelated viruses—AcMNPV and AA V2. System embodiments utilize rep and / or cap genes either stably transfected in cell lines or which are introduced into cells as an expression cassette in a vector. Rep and cap genes that are designed to remain silent until the cell is infected with a viral vector. Infection with viral initiates rescue / amplification of integrated AAV helper genes resulting in dramatic induction of the expression and assembly of rAAV. The arrangement of this specific embodiment provides high levels of Rep and Cap proteins in every cell thus improving rAAV yields by 10-fold. The described vectors are modular in design and may be utilized for the production of other multiprotein complexes.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Inducible system for highly efficient production of recombinant Adeno-associated virus (rAAV) vectors
ActiveUS8679837B2High production costHigh yieldBiocideGenetic material ingredientsClinical gradeDisease
Production of clinical grade gene therapy vectors for human trials remains a major hurdle in advancing cures for a number of otherwise incurable diseases. Disclosed herein are systems based on a stably trans formed insect cell lines harboring helper genes required for vector production. Specifically exemplified are system embodiments that take advantage of DNA regulatory elements from two unrelated viruses—AcMNPV and AA V2. System embodiments utilize rep and / or cap genes either stably transfected in cell lines or which are introduced into cells as an expression cassette in a vector. Rep and cap genes that are designed to remain silent until the cell is infected with a viral vector. Infection with viral initiates rescue / amplification of integrated AAV helper genes resulting in dramatic induction of the expression and assembly of rAAV. The arrangement of this specific embodiment provides high levels of Rep and Cap proteins in every cell thus improving rAAV yields by 10-fold. The described vectors are modular in design and may be utilized for the production of other multiprotein complexes.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Bone mineralization proteins, DNA, vectors expression systems
InactiveUS6858431B2Prevent mineralizationDecrease osteocalcin productionOrganic active ingredientsFungiNucleotideBone formation
The present invention is directed to isolated nucleic acid molecules that encode LIM mineralization protein, or LMP. The invention further provides vectors comprising nucleotide sequences that encode LMP, as well as host cells comprising those vectors. Moreover, the present invention relates to methods of inducing bone formation by transfecting osteogenic precursor cells with an isolated nucleic acid molecule comprising a nucleotide sequence encoding LIM mineralization protein. The transfection may occur ex vivo or in vivo by direct injection of virus or naked plasmid DNA. In a particular embodiment, the invention provides a method of fusing a spine by transfecting osteogenic precursor cells with an isolated nucleic acid molecule having a nucleotide sequence encoding LIM mineralization protein, admixing the transfected osteogenic precursor cells with a matrix and contacting the matrix with the spine. Finally, the invention relates to methods for inducing systemic bone formation by stable transfection of host cells with the vectors of the invention.
Owner:MEDTRONIC SOFAMOR DANEK
LIM mineralization protein splice variants
The present invention is directed to isolated nucleic acid molecules that encode LIM mineralization protein, or LMP. The invention further provides vectors comprising splice variants of nucleotide sequences that encode LMP, as well as host cells comprising those vectors. Moreover, the present invention relates to methods of inducing bone formation by transfecting osteogenic precursor cells with an isolated nucleic acid molecule comprising a nucleotide sequence encoding splice variants of LIM mineralization protein. The transfection may occur ex vivo or in vivo by direct injection of virus or naked plasmid DNA. In a particular embodiment, the invention provides a method of fusing a spine by transfecting osteogenic precursor cells with an isolated nucleic acid molecule having a nucleotide sequence encoding LIM mineralization protein, admixing the transfected osteogenic precursor cells with a matrix and contacting the matrix with the spine. Finally, the invention relates to methods for inducing systemic bone formation by stable transfection of host cells with the vectors of the invention.
Owner:EMORY UNIVERSITY
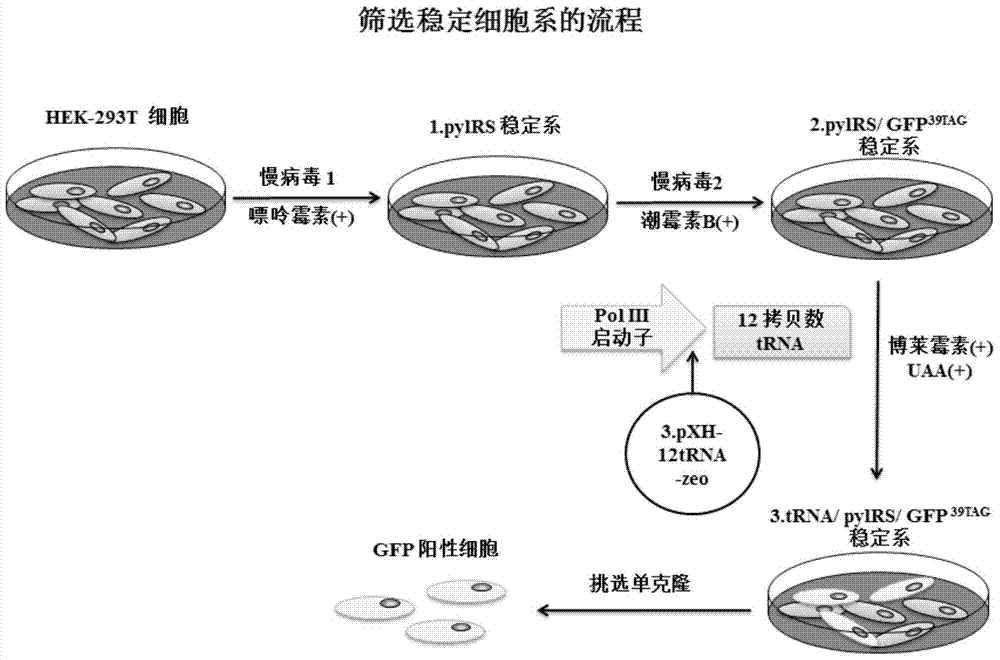
Building of stable cell line carrying orthogonal tRNA/arginyl tRNA synthetase
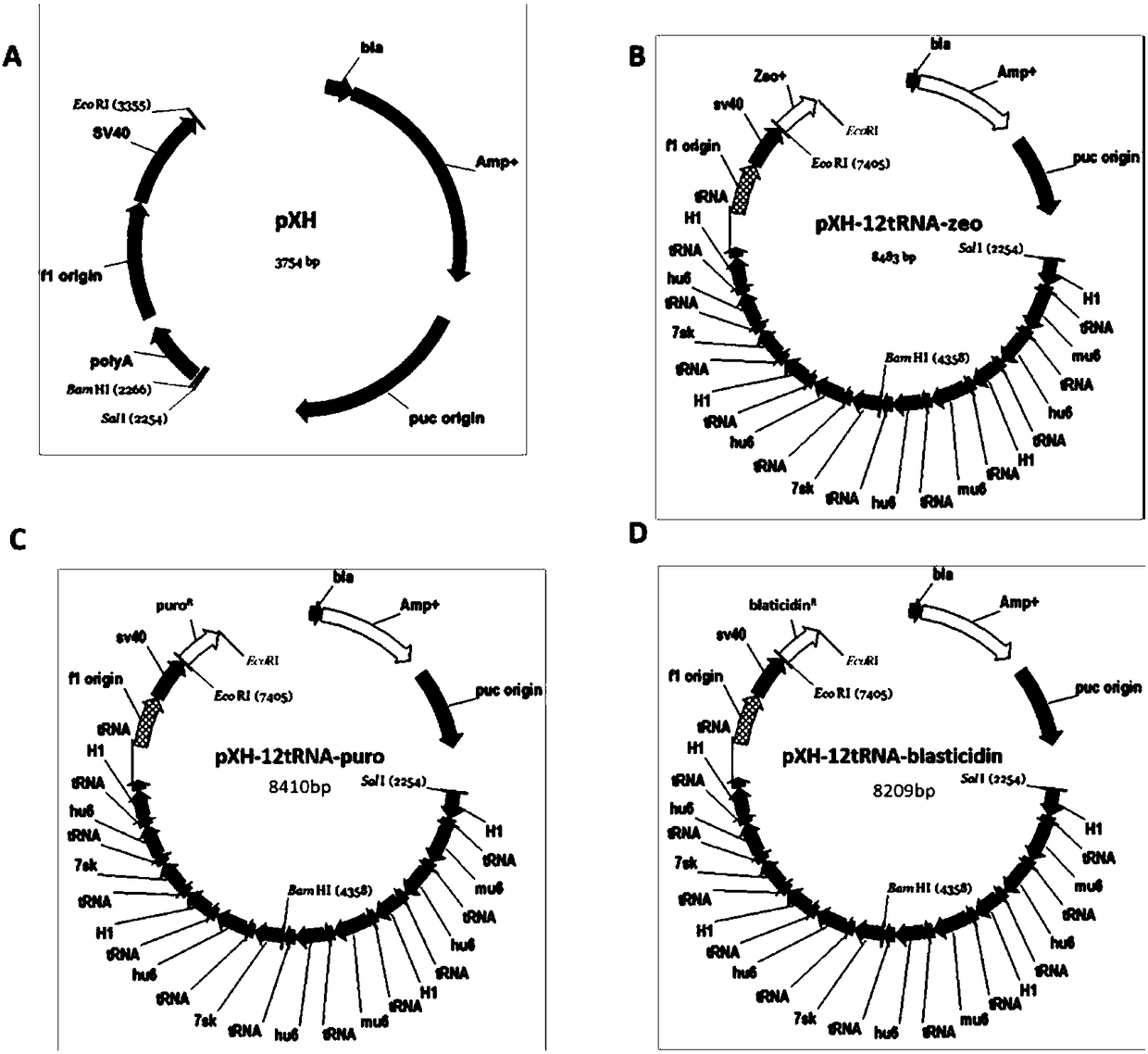
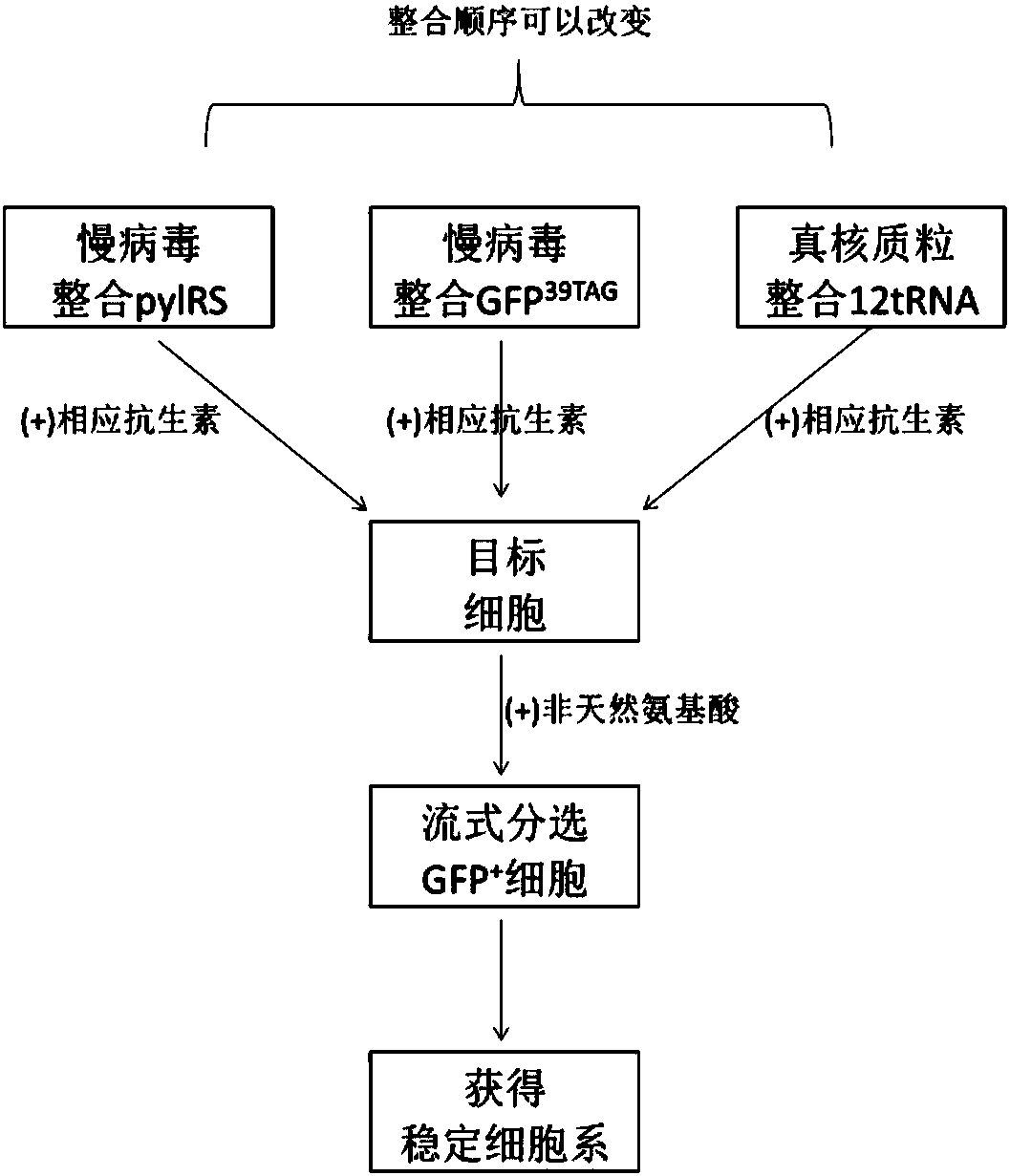
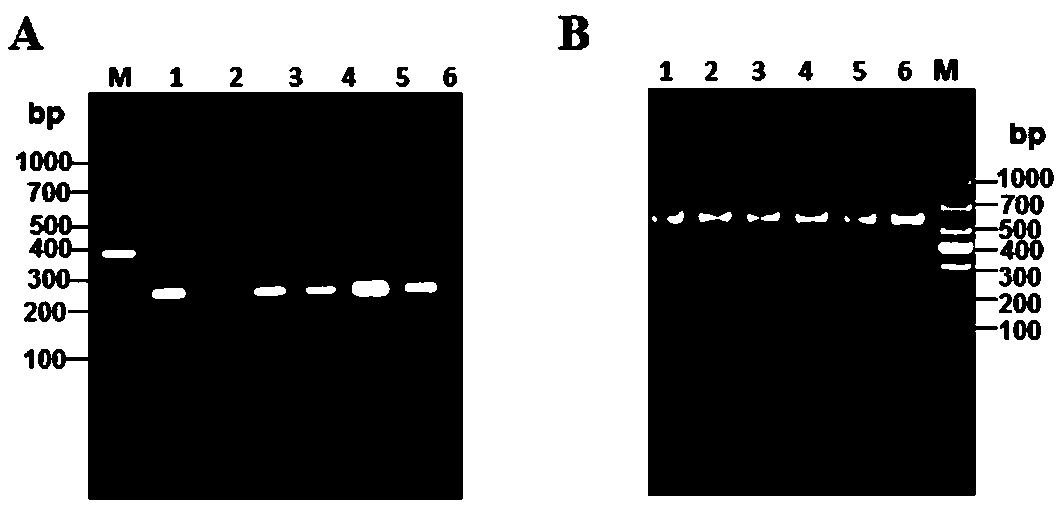
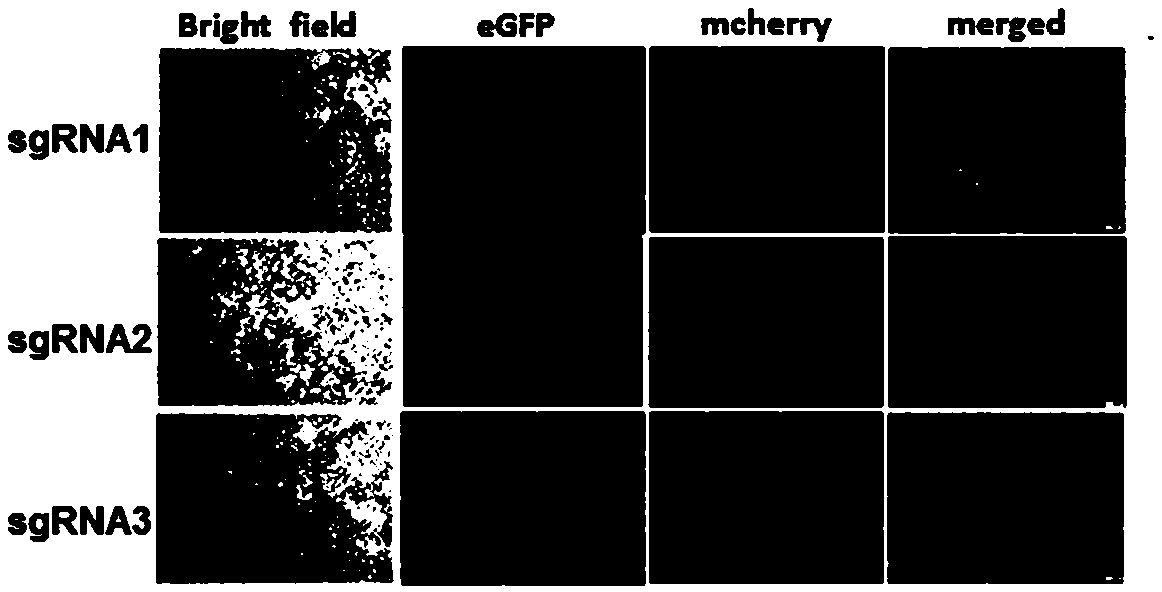
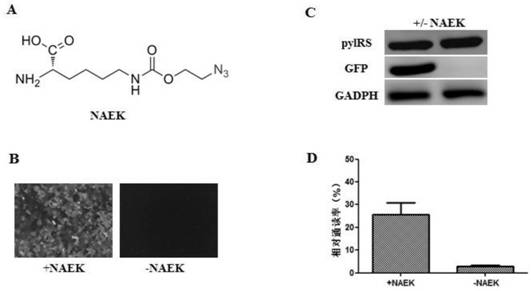
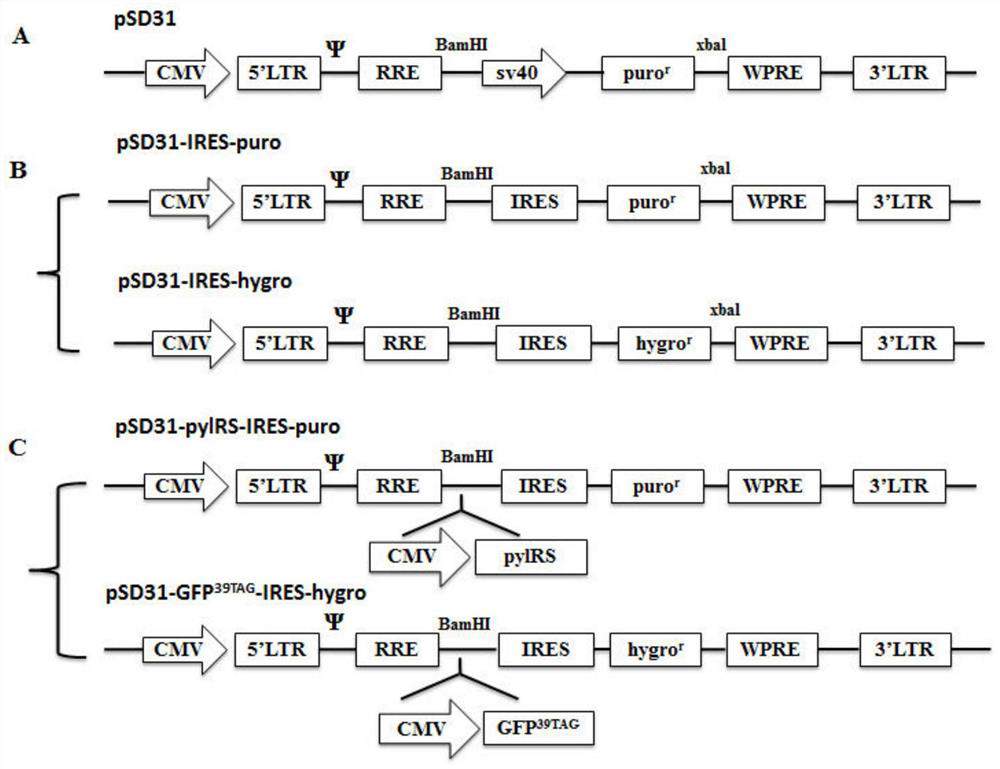
The invention relates to a building method of a stable cell line carrying orthogonal tRNA / arginyl tRNA synthetase. On the basis of a gene codon expansion technology, a pair of orthogonal tRNA / arginyl tRNA synthetase is used for introducing unnatural amino acid into a fixed point of protein. The invention also relates to a building method of double lentiviral vectors, a building method of a multi-copy-number tRNA carrying carrier and a method for stably integrating the orthogonal tRNA / arginyl tRNA synthetase gene into the cell genome through double lentiviral stable transduction and plasmid stable transfection. The invention further relates to application of the stable cell line, such as the purpose of expressing the target protein containingunnatural amino acid.
Owner:PEKING UNIV
Orthotopic transplantation rat liver cancer model and preparing method and application thereof
InactiveCN101185764AGood repeatabilityIncrease success rateIn-vivo testing preparationsLymphatic SpreadRemission rate
The invention pertains to the field of biomedical technology, in particular to an orthotopic transplanted mouse liver cancer model which takes the research of the occurrence and development mechanisms of liver cancer and the development of anti-tumor drugs as the purposes, and the preparation method and the application thereof. The H22 cell strain of stable transfection EGFP is injected into the BALB / c mouse peritoneal cavity for amplification; the ascites is extracted from the H22 ascites cancer mouse peritoneal cavity, then cell separation is carried out and cell suspension is prepared; the mouse peritoneal cavity is opened, and the cell suspension is extracted by a micro-syringe to be injected into the mouse liver; a cotton bud with 75 percent alcohol is pressed on the needle hole till the surface of the liver does not bleed any more, and the needle hole is sealed by bonding agent; then the liver surface is washed by physiological saline, and layer separation is carried out and the peritoneal cavity is closed. The results show that the occurrence rate of mouse liver cancer is 100 percent and the natural remission rate is zero. The model is an ideal orthotopic liver cancer model which can be used in the research of the development and metastasis mechanisms of orthotopic liver cancer and can also be applicable to the development of anti-tumor drugs, the experimental treatment and diagnosis research of liver cancer.
Owner:NANJING UNIV
Method of inducing differentiation of neural stem cell into dopaminergic neuron by using recombinant slow virus
ActiveCN103865956AMultiple differentiation potentialHigh expressionNervous system cellsFermentationSingle cell suspensionApoptosis
The invention relates to bioengineering technologies, and particularly relates a method of inducing differentiation of neural stem cells into dopaminergic neurons by using recombinant slow virus. The method comprises the following steps: constructing a recombinant slow virus vector; separating, culturing and identifying the neural stem cells; transfecting the recombinant slow virus into NSCs; identifying the NSCs transfected by the recombinant slow virus, wherein the process of transfecting the recombinant slow virus into the NSCs comprises the following steps: digesting NSCs after being subjected to three times of passages to prepare a single-cell suspension, and inoculating into a culture plate; transfecting the TH+Brn4 gene recombinant slow virus; adding the slow virus and ploybrene, uniformly shaking, putting the culture plate into an incubator to culture for 1-2 hours, and replacing fresh differential culture medium; meanwhile, screening by using puromycin to obtain stable transfected cells. The method has the advantages as follows: by constructing the slow virus vectors of target genes TH and Brn4, exogenous genes after transfecting the NSCs can be stably expressed in cells; high proportion of TH positive dopaminergic neuron is generated after differentiation; the Brn4 can promote high expression of neurotrophic factors and has the function of inhibiting apoptosis.
Owner:HELP STEM CELL INNOVATIONS CO LTD
Antineoplastic invasion transfer function of snake venom metalloprotease inhibitors BJ46a and uses thereof
InactiveCN101429525AInhibitory activityObvious anti-tumor invasion and metastasis effectPeptide/protein ingredientsGenetic material ingredientsAbnormal tissue growthIn vivo
The invention discloses a snake venom metal protease inhibitor BJ46a capable of resisting attack and transference of tumor and application thereof, and belongs to the field of medical organism. In the invention, according to the BJ46a gene sequence (AF294836) in GenBank, the BJ46a full gene is designed and synthesized. An expression vector cloned to baculovirus can generate the recombined BJ46a protein capable of inhibiting the activity of the substrate metal protease in Sf9 insect cells, and in vivo and in vitro experiments show that the BJ46a protein has the functions of resisting the attack and transference of melanoma cells B16. The inhibitor adopts the genetic transfection technique, and can establish B16 / pcDNA3.1HisC-BJ46a cell strains with stable transfection, and the in vivo and in vitro experiments prove that the BJ46a can inhibit the attack and transference of B16 cells at the gene level. The inhibitor is applied to preventing and treating the attack and transference of tumor, and has great application prospect.
Owner:FUJIAN MEDICAL UNIV
Lentiviral plasmid expression vector as well as construction method and application of lentiviral plasmid expression vector
InactiveCN104017827AImprove the level of clinical diagnosis and treatmentImprove the level of diagnosis and treatmentBiological testingFermentationMuscle specific receptor tyrosine kinase AntibodyStaining
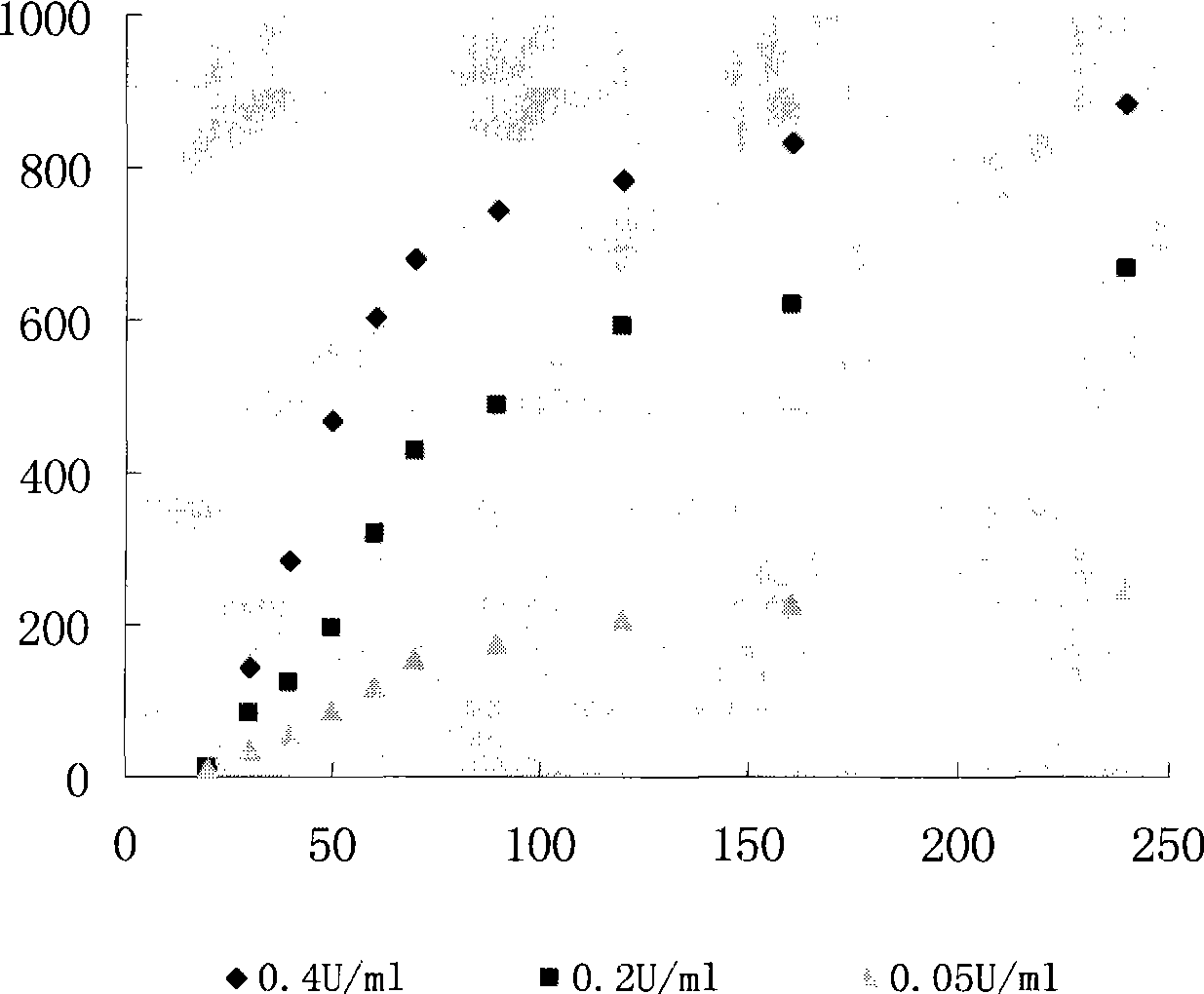
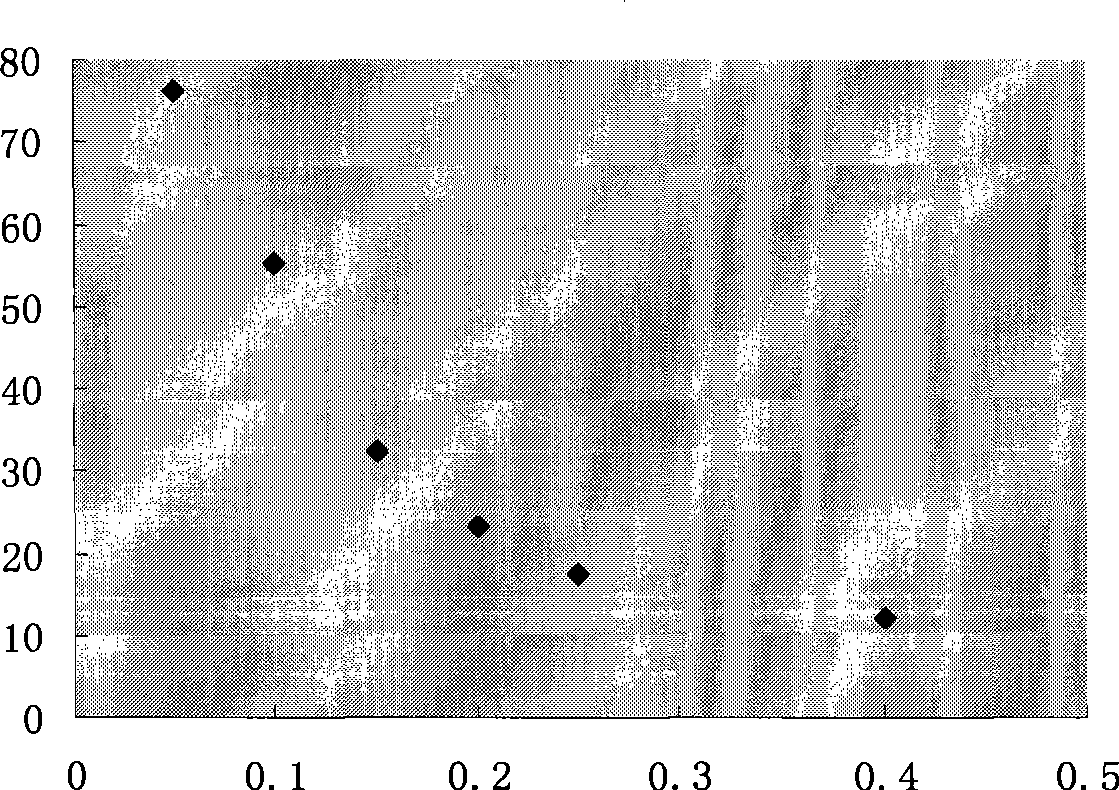
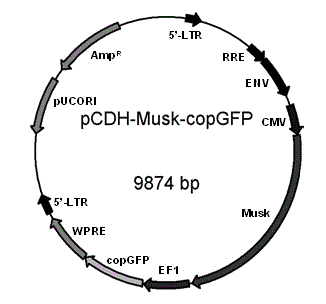
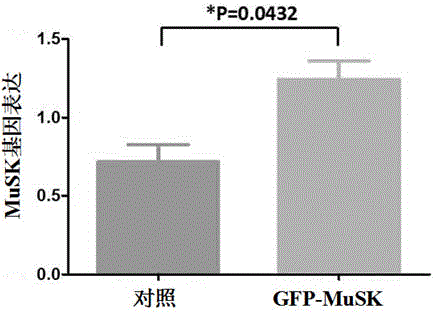
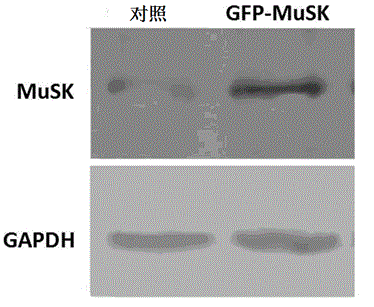
The invention discloses a lentiviral plasmid expression vector as well as a construction method and an application of the lentiviral plasmid expression vector. The lentiviral plasmid expression vector is pCDH-MCS-MuSK-GFP and the nucleotide sequence of the lentiviral plasmid expression vector is shown in SEQ ID NO. 2 in a sequence table. The invention further discloses an application of the lentiviral plasmid expression vector in construction of an anti-muscle-specific receptor tyrosine kinase antibody detection. According to the invention, a stable transfected cell line of MuSK is established by constructing a viral vector, a MuSK antibody is qualitatively and quantitatively detected clinically by an immunofluorescence staining method with relatively high sensitivity and specificity in combination with a flow cytometry, the detection results are more stable and manpower and time costs are greatly saved. The diagnosis and treatment levels of myasthenia gravis are greatly improved by the establishment of the project, the labor capacity and life quality of patients are improved and the lentiviral plasmid expression vector has significant social and economic benefits.
Owner:GENERAL HOSPITAL OF TIANJIN MEDICAL UNIV
Method for suspension culture of sensitive cells and method for producing blue ear disease vaccine by using sensitive cells
InactiveCN102453699AViral antigen ingredientsMicrobiological testing/measurementDiseaseCell adhesion
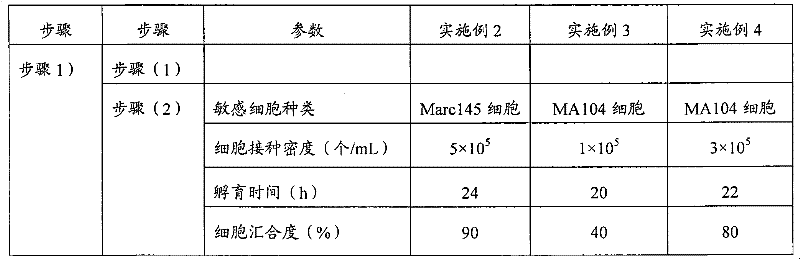
The invention provides a method for suspension culture of sensitive cells, which comprises the following steps that: (1) expression vectors containing gene sequences, capable of weakening the cell adhesion performance, are used for stably transfecting Marc145 cells or MA104 cells; (2) a stable transfection cell clone is screened and separated; (3) the gene expression in the stable transfection cell clone is detected; and (4) the stable transfection cell clone is subjected to suspension culture. The method further relates to a method for producing blue ear disease vaccine vaccine by using the Marc145 cells or MA104 cells obtained through the suspension culture.
Owner:BEIJING SKYWING TECH CO LTD
Novel IL23 antagonist
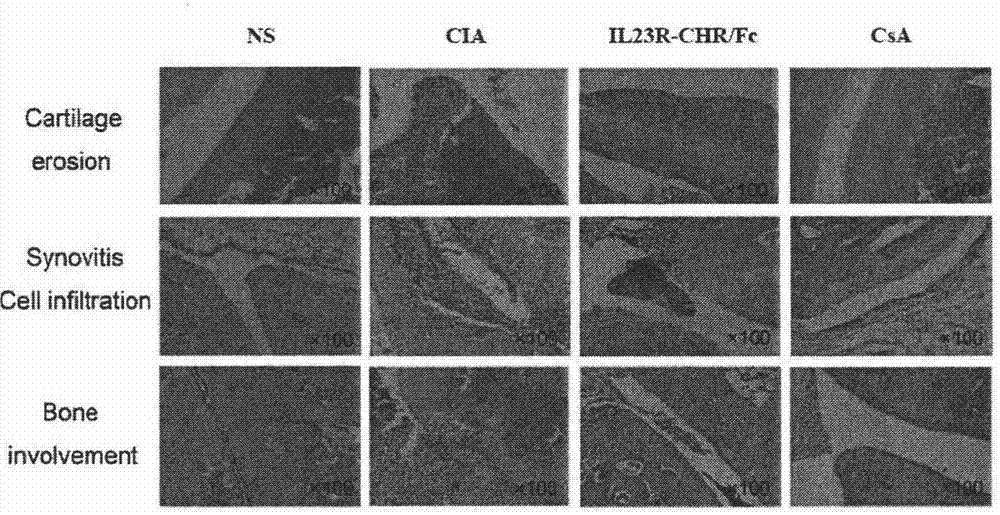
The invention relates to a recombinant human IL (Interleukin)-23 receptor extracellular region / Fc fusion protein capable of combining with IL-23 and antagonizing a function of IL-23. The fusion protein is characterized in that a fusion protein encoding gene is rich in mammalian cell preferred codons, and is highly expressed by a CHO (Chinese Hamster Ovary) stable transfection cell strain. The protein is formed by fusion of a gene optimized IL-23R-CHR active fragment and a human immune globulin molecule Fc fragment; the defects that the half-life period of the prokaryotic expression IL-23R-CHR fragment is short, the stability is poor and endotoxin is difficult to remove are overcome; the biological activity of IL-23-CHR is reserved; in addition, the fusion protein does not have ADCC (Antibody Dependent Cellular Cytotoxicity) and CDC (Complement Dependent Cytotoxicity) effects, so that the fusion protein is more suitable for treatment of chronic diseases such as an autoimmune disease and a chronic infection; and the contents of molecular design, expression, purification, construction of a stable expression cell strain and disease treatment and the like of the fusion protein are included.
Owner:CHINA PHARM UNIV
Method for suspension culture of subculture cells and method for producing hog cholera vaccine by using subculture cells
InactiveCN102453698AViral antigen ingredientsMicrobiological testing/measurementCell adhesionAdhesion process
The invention provides a method for suspension culture of subculture cells, which comprises the following steps that: (1) expression vectors containing gene sequences, capable of weakening the cell adhesion property, are used for stably transfecting pig testis cells or pig kidney cells; (2) a stable transfection cell clone is screened and separated; (3) the gene expression in the stable transfection cell clone is detected; and (4) the stable transfection cell clone is subjected to suspension culture. The method further relates to a method for producing hog cholera vaccine by using the pig testicle cells or the pig kidney cells obtained through the suspension culture.
Owner:BEIJING SKYWING TECH CO LTD
Autoimmune encephalitis antibody transient transfection and stable transfection detection method and application thereof
PendingCN113755443ABiological material analysisMicroorganism based processesImmunofluorescenceTransient transfection
The invention provides an autoimmune encephalitis antibody transient transfection and stable transfection detection method and application thereof. Specifically, the invention provides a recombinant cell for expressing mutant NMDAR, the recombinant cell expresses fusion protein of exogenous mutant NMDAR and fluorescent protein, and the transfection survival rate is high. The recombinant cell provided by the invention can be used for detecting antibodies related to autoimmune encephalitis, including NMDAR, AMPAR1, AMPAR2, LGI1, Caspr2 and GABABR. The invention also provides a method for detecting anti-autoimmune encephalitis by using the transfected cell line through an indirect immunofluorescence method based on the recombinant transfected cell line provided by the invention. The method disclosed by the invention has high sensitivity and specificity, and can be used for accurately detecting the types of pathogenic antibodies so as to help a patient to carry out targeted immunotherapy and recover health.
Owner:陈向军
Methods and Compositions for Generating Stable Transfected Cells
Methods and compositions are provided involving high producing cell lines. Embodiments concern efficient methods for screening for such cell lines and for creating such cell lines. These cell lines can be used to create large amounts of protein. To quickly generate large quantity of recombinant proteins or vaccines for both pre-clinical study and clinical trials, almost all drug development will face the same challenging obstacle of rapidly generating a high stable producer. Developing and identifying a stable cell line is a critical part of biopharmaceutical development.
Owner:MAXCYTE
Construction of stable cell line carrying orthogonal tRNA/aminoacyl tRNA synthetase
InactiveCN109295100AAchieve stable expressionStable expressionPeptide/protein ingredientsGenetically modified cellsAminoacyl-tRNA synthetasesAminoacyl-tRNA
The present invention relates to a method for constructing a stable cell line carrying an orthogonal tRNA / aminoacyl tRNA synthetase, and the method stably integrates an orthogonal tRNA / aminoacyl tRNAsynthetase gene into cell genomes by double lentiviral stable transduction and plasmid stable transfection. The invention further relates to the stable cell line obtained by the method and the use ofthe stable cell line in the expression of a protein containing non-natural amino acids, and by utilizing unique reactive groups on the non-natural amino acids, the purpose of high-efficiency and specific protein modification can be achieved.
Owner:PEKING UNIV
Mouse beta-alexin 1 recombinant plasmids, polypeptides, uses and preparations thereof
InactiveCN101353668ACan't produce drug resistanceAvoid fusesPeptide/protein ingredientsGenetic material ingredientsEukaryotic plasmidsGene engineering
Owner:SICHUAN UNIV
Fructus lycii arabinogalactan as well as preparation method and application thereof
ActiveCN109369815AHas the effect of treating Alzheimer's diseaseNervous disorderFood ingredient functionsPurification methodsPotential effect
The invention relates to a polysaccharide LBP1a1-1 extracted from fructus lycii, application of the polysaccharide in preparation of medicines or health care products for preventing and / or treating neurodegenerative diseases, and application of the polysaccharide in preparation of medicines or health care products for inhibiting Abeta42 and in preparation of medicines or health care products for preventing and treating Alzheimer's disease. Specifically, the invention relates to arabinogalactan extracted from the fructus lycii. The preparation method comprises the following steps: extracting crude polysaccharides in the fructus lycii by combining cellulase, amylase and papain with water of 55 DEG C, and performing alcohol precipitation; and identifying by combining multiple column chromatography purification methods with spectrum analysis, thereby obtaining the arabinogalactan. In vitro experiments prove that the polysaccharide is capable of inhibiting production of the Abeta42 in CHO / APPBACE1 cells for stable transfection of APP and BACE1 in a dosage dependent manner. Therefore, the polysaccharide has a potential effect of treating the Alzheimer's disease, and is expected to be developed into a carbohydrate drug for treating the Alzheimer's disease.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
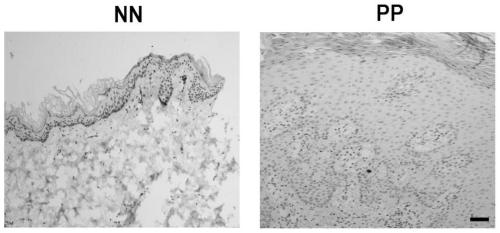
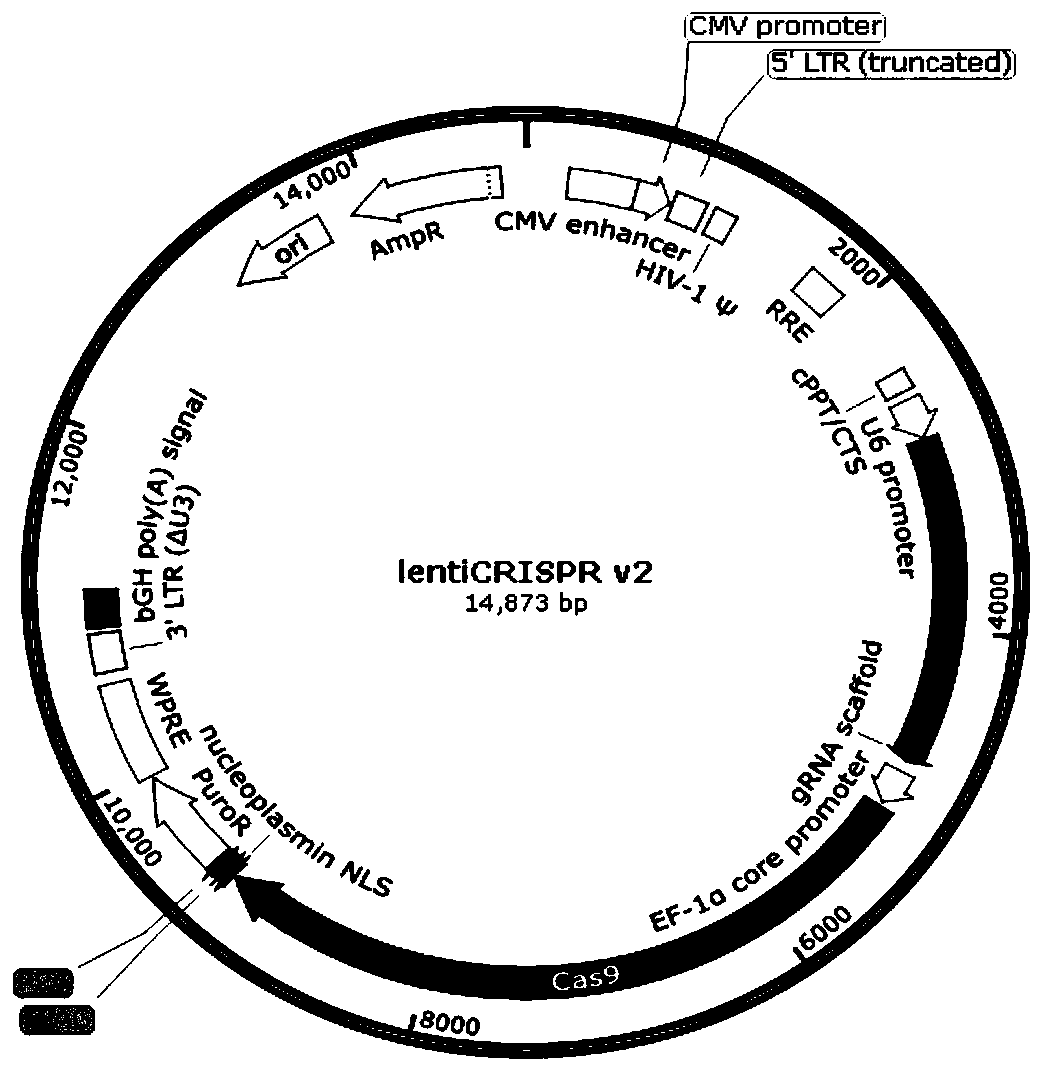
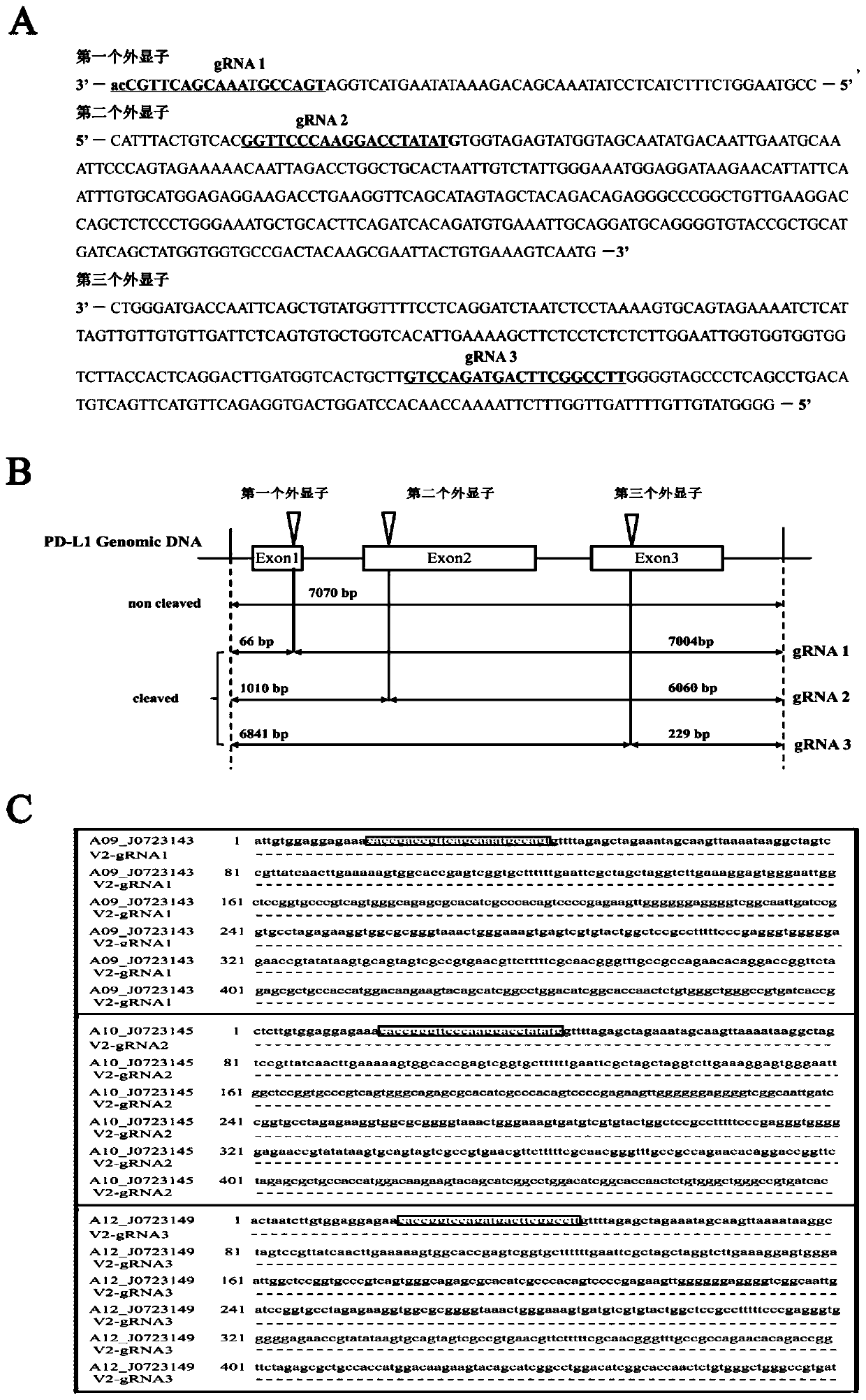
Method for knocking out PD-L1 gene in keratinocytes by CRISPR/Cas9 gene editing method
The invention relates to the field of cell model construction, and concretely relates to a method for knocking out a PD-L1 gene in keratinocytes by a CRISPR / Cas9 gene editing method, and a keratinocyte model constructed by the method and an application thereof. The method has the advantages that the PD-L1 knockout keratinocyte stable-transfection strain constructed by the invention can be used not only for the research of psoriasis, but also for the research of other skin diseases with low expression of PD-L1. The construction of the PD-L1 knockout cell strain is helpful for exploring the role of PD-L1 in skin-related diseases such as psoriasis, pityriasis rosea, and allergic contact dermatitis, and further explores the function of PD-L1 in keratinocytes.
Owner:SHANGHAI CHANGHAI HOSPITAL
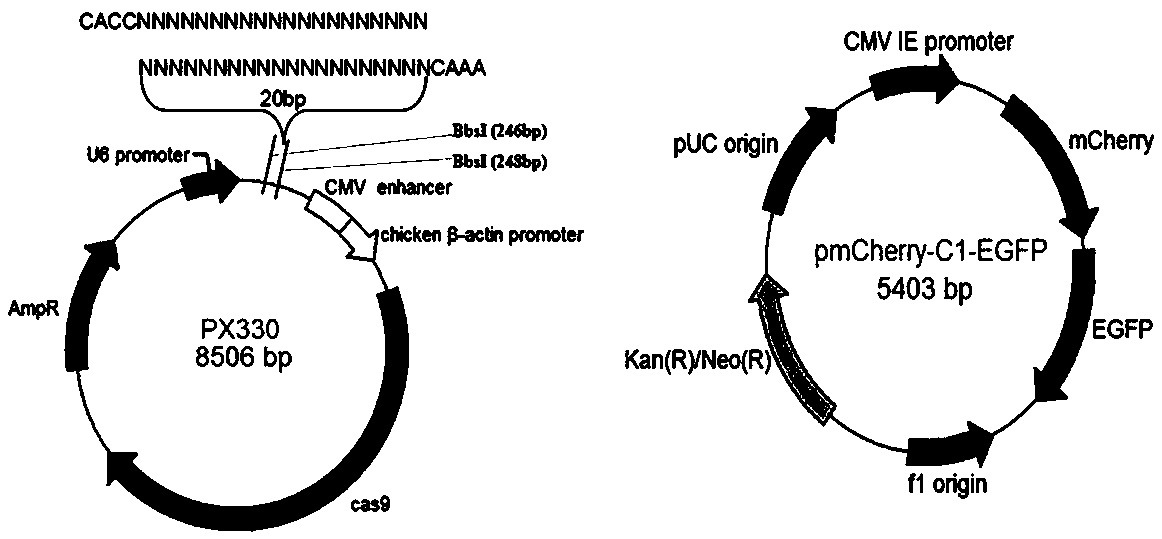
Monoclonal cell culture method
ActiveCN108130314AImprove survival rateGood for follow-up experimentsStable introduction of DNACell culture supports/coatingMatrigelGenome editing
The application of the invention belongs to the technical field of culture of monoclonal cells, and particularly relates to the application matters of the invention of a monoclonal cell culture methodin the process of constructing a stable expression cell system through the gene editing technology. The method takes the gene editing technology CRISPR / Cas9 as the basis. The method specifically comprises the following steps: editing a genome through the CRISPR / Cas9 technology, and culturing a screened cell through a 3D soft fibrous protein matrigel. In a word, the culture condition of the optimized 3D soft fibrous protein matrigel is used to construct a monoclonal cell population, the survival rate of the single cell is greatly increased, and the stable transfection cell system sharing identical gene modification and identical genetic background is rapidly screened. Particularly for the cell difficult to growth through the common culture method, the monoclonal cell culture method has obvious advantages and is favorable for the implementation of the follow-up experiments.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Construction of stable cell line carrying orthogonal tRNA/aminoacyl tRNA synthetases
InactiveCN112725282AGenetically modified cellsMicroorganism based processesStable cell lineAmino acid
The invention relates to a construction method of a stable cell line carrying orthogonal tRNA / aminoacyl tRNA synthetases. On the basis of a gene codon extension technology, a pair of orthogonal tRNA / aminoacyl tRNA synthetases is used for introducing non-natural amino acid into protein at a fixed point. The invention also relates to a construction method of a double lentiviral vector, a construction method of an orthogonal tRNA carrier carrying multiple copy numbers and a method for stably integrating orthogonal tRNA / aminoacyl tRNA synthetase genes into a cell genome by virtue of stable transduction of double slow viruses and stable transfection of plasmids. The invention further relates to the use of the stable cell line, such as the use for the expression of interest proteins containing non-natural amino acids.
Owner:PEKING UNIV
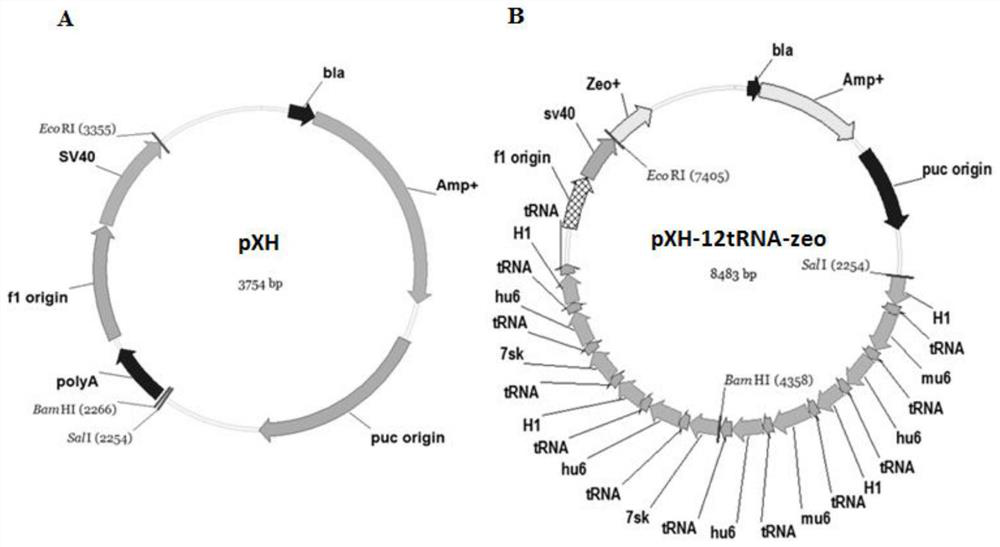
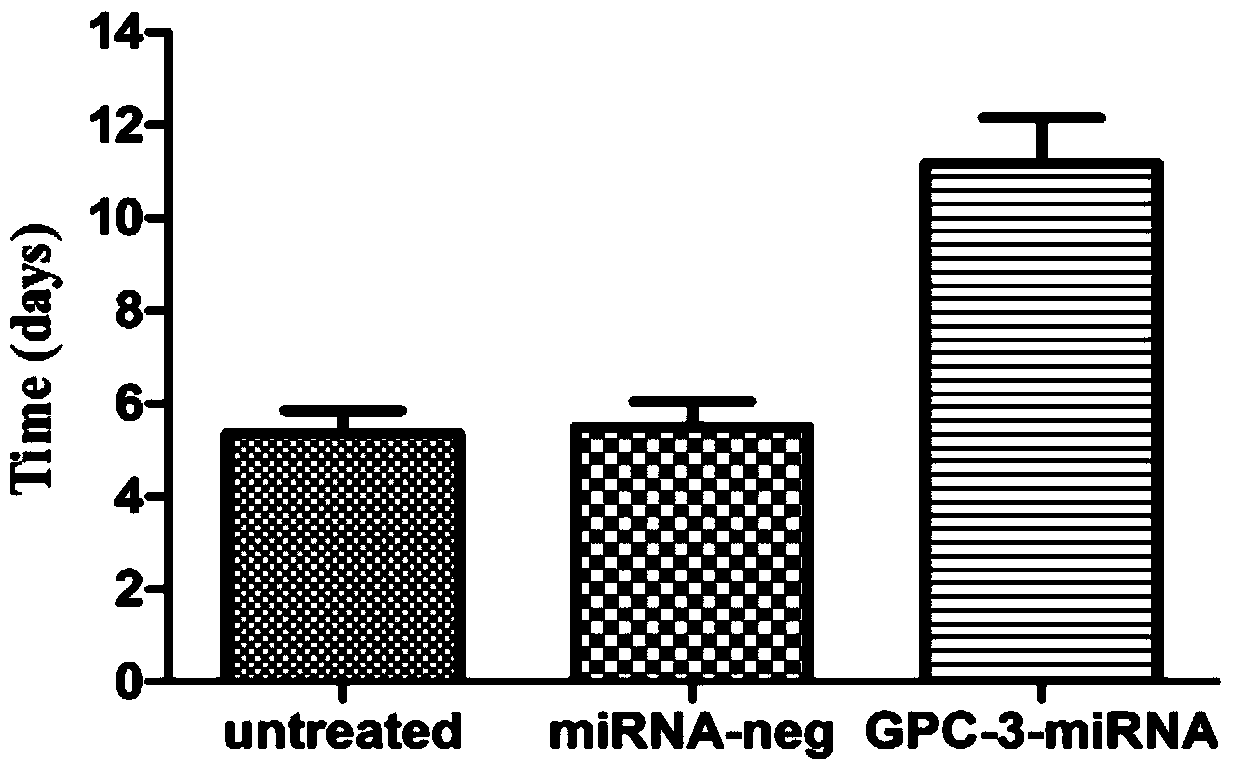
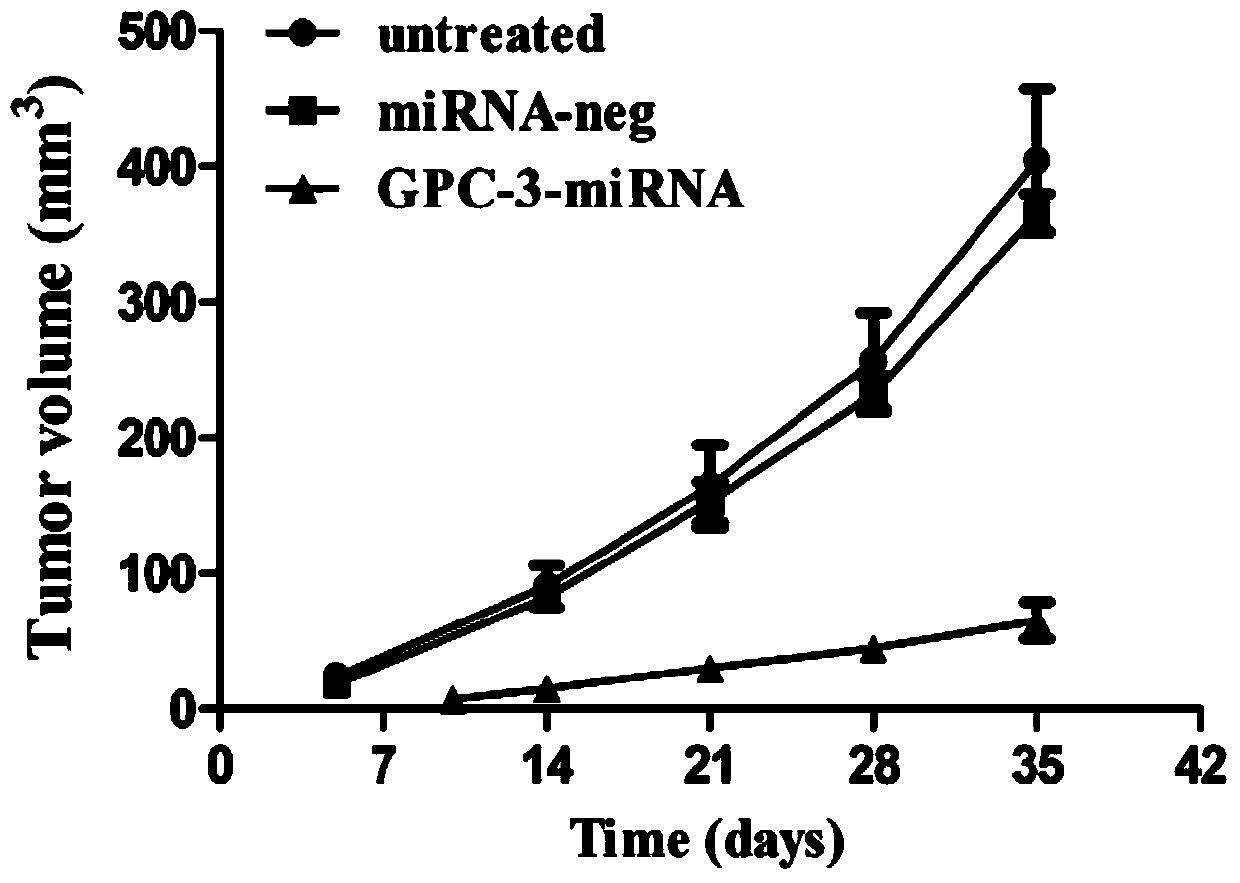
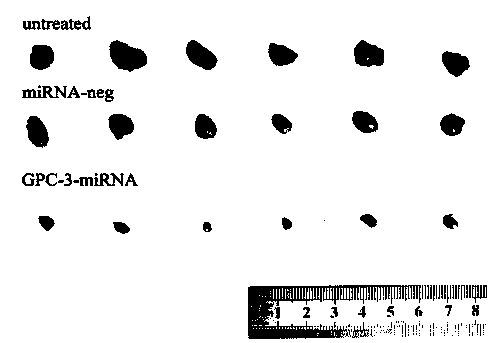
Method for detecting inhibiting ability of silent GPC-3 gene transcription on hepatocellular carcinoma transplanted tumor in nude mouse
InactiveCN103743902ASimple methodVector-based foreign material introductionMaterial analysisFluorescenceHepg2 cells
The invention discloses a method for detecting the inhibiting ability of silent GPC-3 (glypican-3) gene transcription on a hepatocellular carcinoma transplanted tumor in a nude mouse. The method includes: 1) designing and synthesizing 4 pairs of miRNA oligomeric single-strand DNA specific to a target gene consensus sequence, then synthesizing the corresponding dsDNA, inserting the dsDNA into a vector to construct 4 recombinant plasmids, and picking out the recombinant plasmid with the highest interference efficiency through fluorescence quantitative PCR; 2) transfecting the recombinant plasmid with the highest interference efficiency into HepG2 cells to construct transfected HepG2 cells, and performing screening, thus obtaining a stably transfected HepG2 cell; 3) inoculating the stably transfected HepG2 cell to the nude mouse subcutaneously to construct a human hepatocellular carcinoma tumor-bearing nude mouse model; and 4) then determining the growth state of the stably transfected HepG2 cell in the human hepatocellular carcinoma tumor-bearing nude mouse model. The method provided by the invention can be employed to detect the inhibiting ability of silent GPC-3 gene transcription on a hepatocellular carcinoma transplanted tumor in the nude mouse, and can be used for research of GPC-3 on transplanted tumors.
Owner:AFFILIATED HOSPITAL OF NANTONG UNIV
Chicken interferon alpha biological activity detection method
InactiveCN108795982AShorten detection timeAvoid Biosafety HazardsCompound screeningApoptosis detectionPromoter activityNeomycin
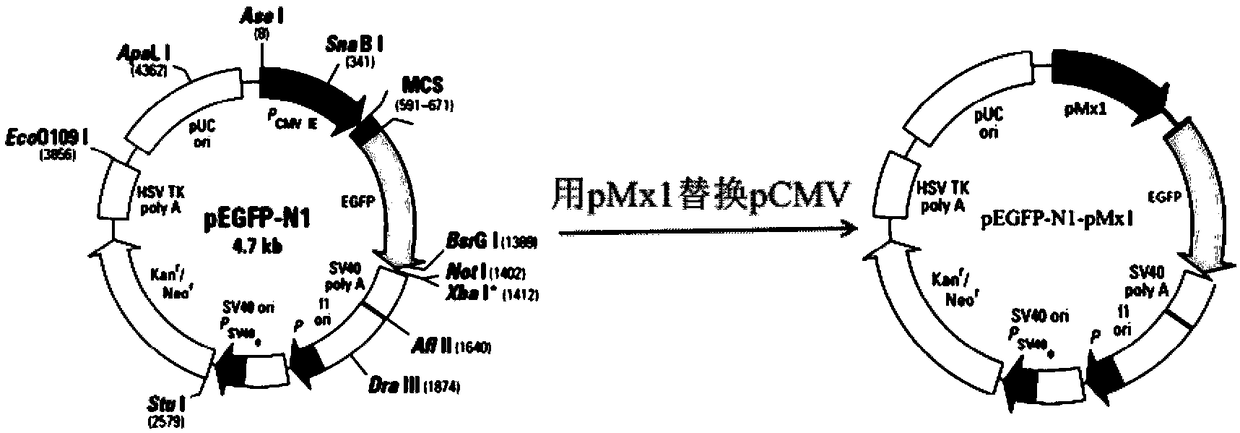
The invention discloses a chicken interferon alpha biological activity detection method, and applications thereof. The chicken interferon alpha biological activity detection method comprises followingsteps: PCR amplification is adopted to obtain Mxp gene segments of chicken Mx protein; pCMV of pEGFP-N1 vector plasmid is removed; the Mxp gene segments of chicken Mx protein obtained through PCR amplification are subjected to construction of pEGFP-N1-Mxp plasmid through replacing of pCMV of orginal pEGFP-N1 vector plasmid with T4DNA ligase; the pEGFP-N1-Mxp plasmid is adopted for cell transfection, and a cell strain capable of realizing stable transfection is obtained through screening using neomycin; the screened cell strain capable of realizing stable transfection is subjected to cloning culture, chicken interferon alpha is added for co-incubation with the cell strain which is capable of realizing stable transfection and is treated throug cloning culture, so that Mx gene promoter activity is activated to promote intracellular EGFP expression, the intensity of fluorescence emitted by cells after excitation light source irradiation is positively related to chicken interferon alpha biological activity, so that quantitative evaluation of chicken interferon alpha biological activity is realized.
Owner:ANHUI JIUCHUAN BIOTECH
Tetracycline stringent controlled eucaryon expression vector
InactiveCN101481704ARepress transcriptionTranscription releaseVector-based foreign material introductionFluorescenceHost genome
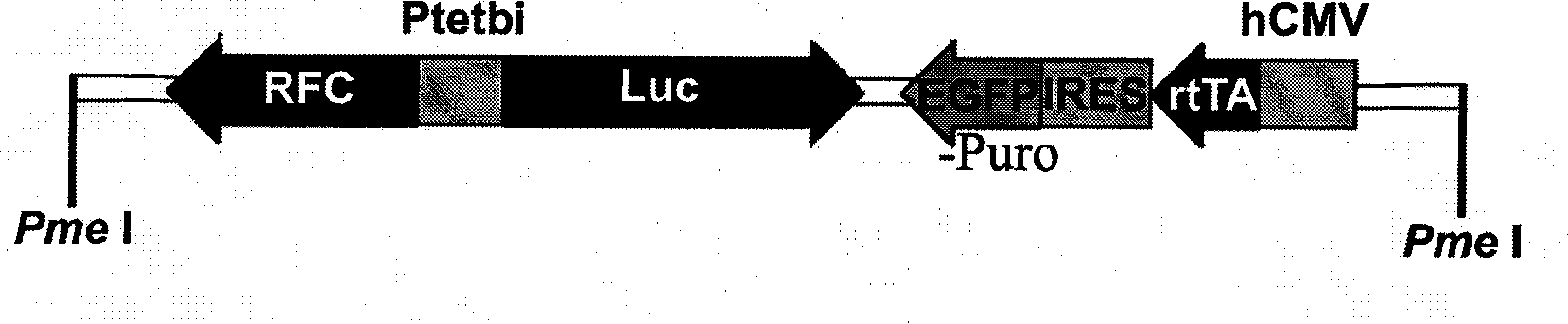
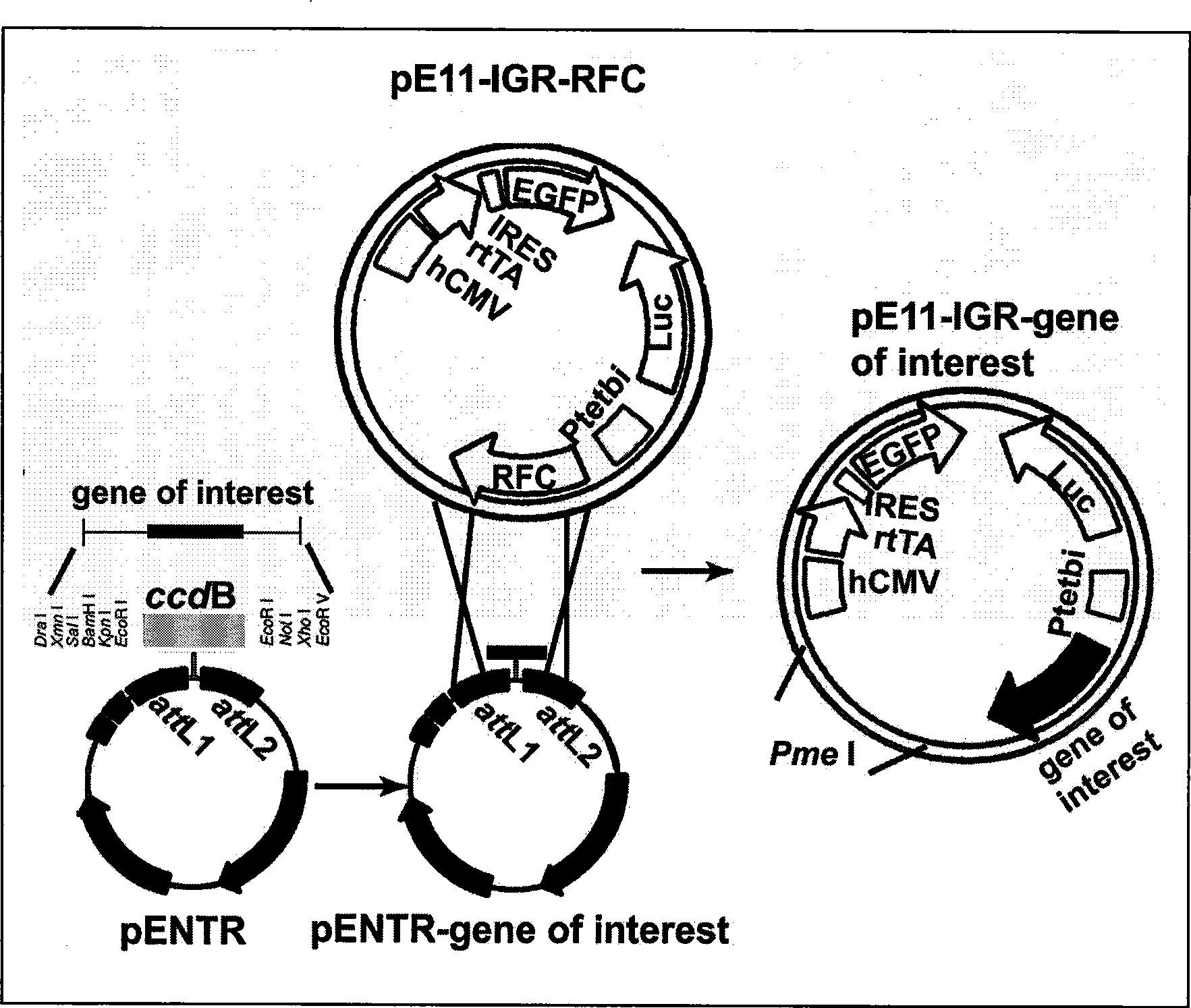
The invention provides a tetracycline strictly controlled eukaryotic expression vector. Following DNA elements including hCMV, rtTA2-M2, IRES, EGFP-Puro, element (Luc) of coding luciferase and Ptetbi-RFC are respectively introduced and are used for the expression and screening of exogenous interest genes in eukaryotic cells. The vector is strictly controlled by Tet or Dox after being transfected. And the expression level of the genes is quantitatively regulated through the amount of the added Tet or Dox. The expression level of the interest genes can be indirectly detected through the detection of the activity of Luc luciferase. The transfection efficiency of the vector can be completed through the detection of the proportion of green fluorescyte. The stably transfected cells are sorted by a flow cytometry. Thus, the expression of the interest genes is ensured to be simple and convenient. And in a stable cell line obtained through screening, the interest genes are integrated with a host genome. The tetracycline strictly controlled eukaryotic expression vector has long stable expression characteristics and advantages.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
LIM mineralization protein splice variants
The present invention is directed to isolated nucleic acid molecules that encode LIM mineralization protein, or LMP. The invention further provides vectors comprising splice variants of nucleotide sequences that encode LMP, as well as host cells comprising those vectors. Moreover, the present invention relates to methods of inducing bone formation by transfecting osteogenic precursor cells with an isolated nucleic acid molecule comprising a nucleotide sequence encoding splice variants of LIM mineralization protein. The transfection may occur ex vivo or in vivo by direct injection of virus or naked plasmid DNA. In a particular embodiment, the invention provides a method of fusing a spine by transfecting osteogenic precursor cells with an isolated nucleic acid molecule having a nucleotide sequence encoding LIM mineralization protein, admixing the transfected osteogenic precursor cells with a matrix and contacting the matrix with the spine. Finally, the invention relates to methods for inducing systemic bone formation by stable transfection of host cells with the vectors of the invention.
Owner:EMORY UNIVERSITY
Cytochrome c protein and assay
InactiveUS20090053748A1Fluorescence enhancementReduced expression levelBacteriaFermentationCytochrome cLiving cell
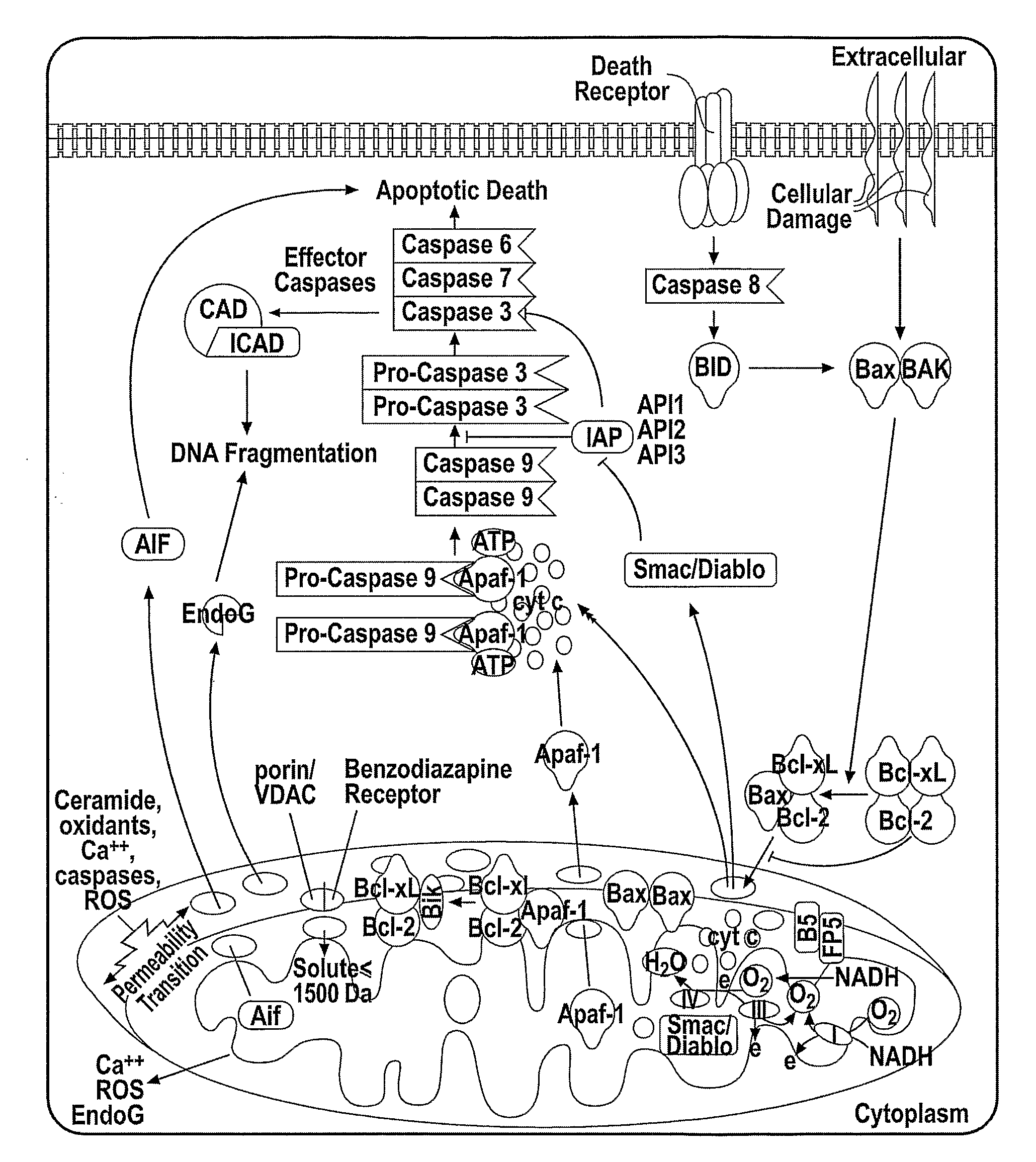
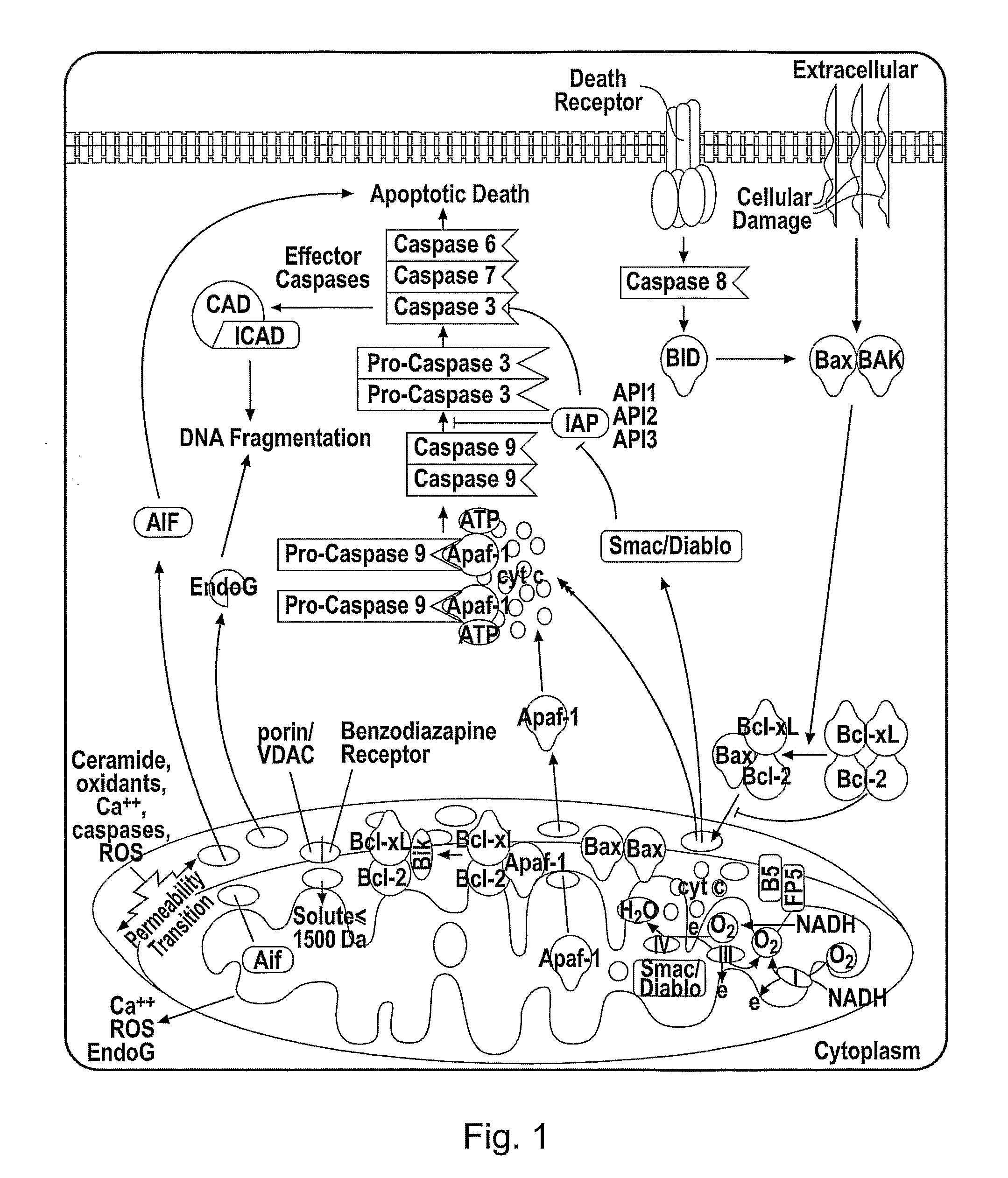
The present invention relates to a a cytochrome c-reporter fusion protein construct comprising a modified cytochrome c protein which targets the mitochondria and has a reduced ability to induce apoptosis in a living cell. The invention also relates to nucleic acid constructs encoding such protein fusions and cells stably transfected with such constructs. The stably transfected cells of the invention can be used in assays to detect apoptosis.
Owner:GE HEALTHCARE LTD
Functional Screening Assay
InactiveUS20070292898A1Determine effectMicroorganism preservationBiological testingFluorescenceNucleotide
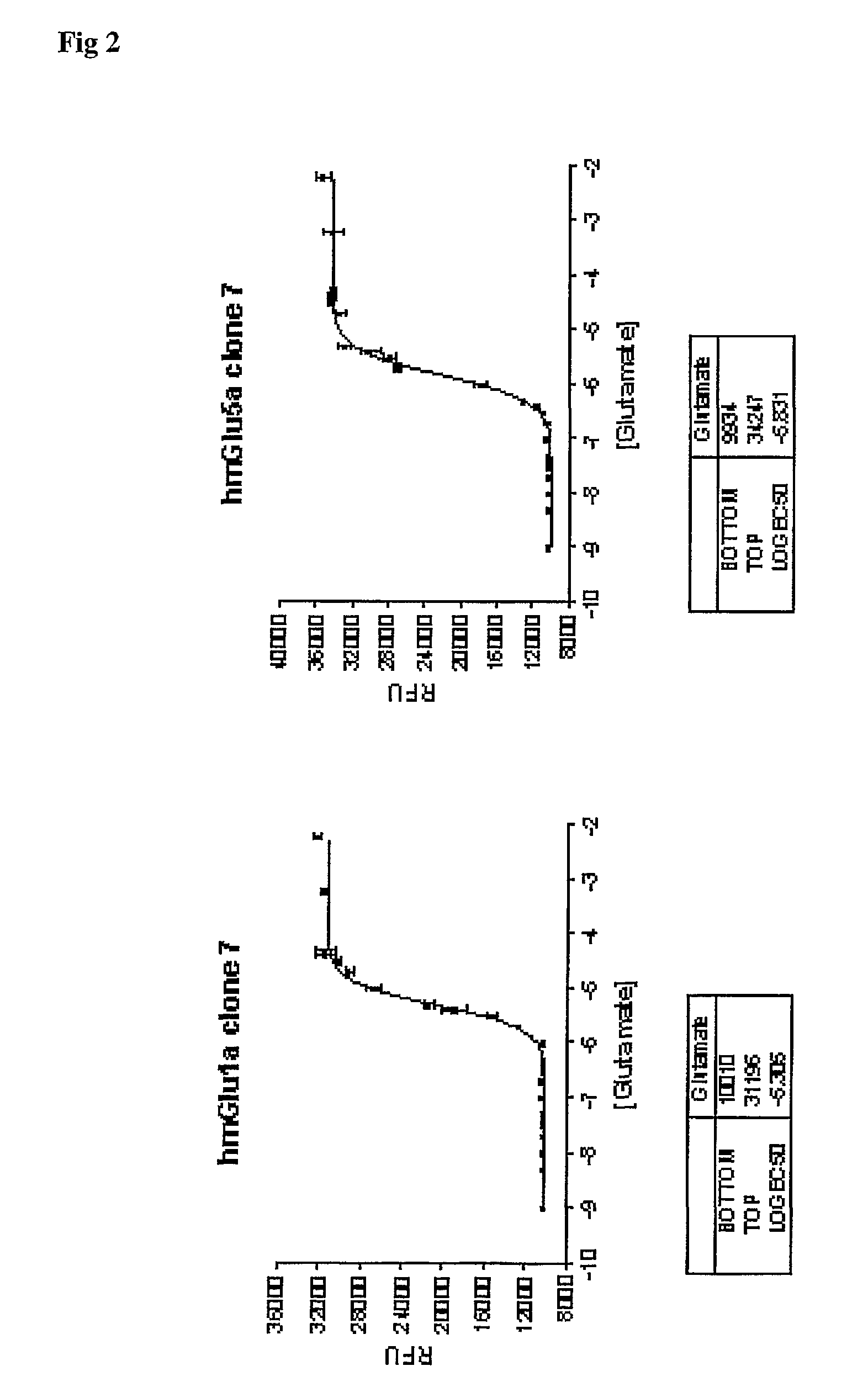
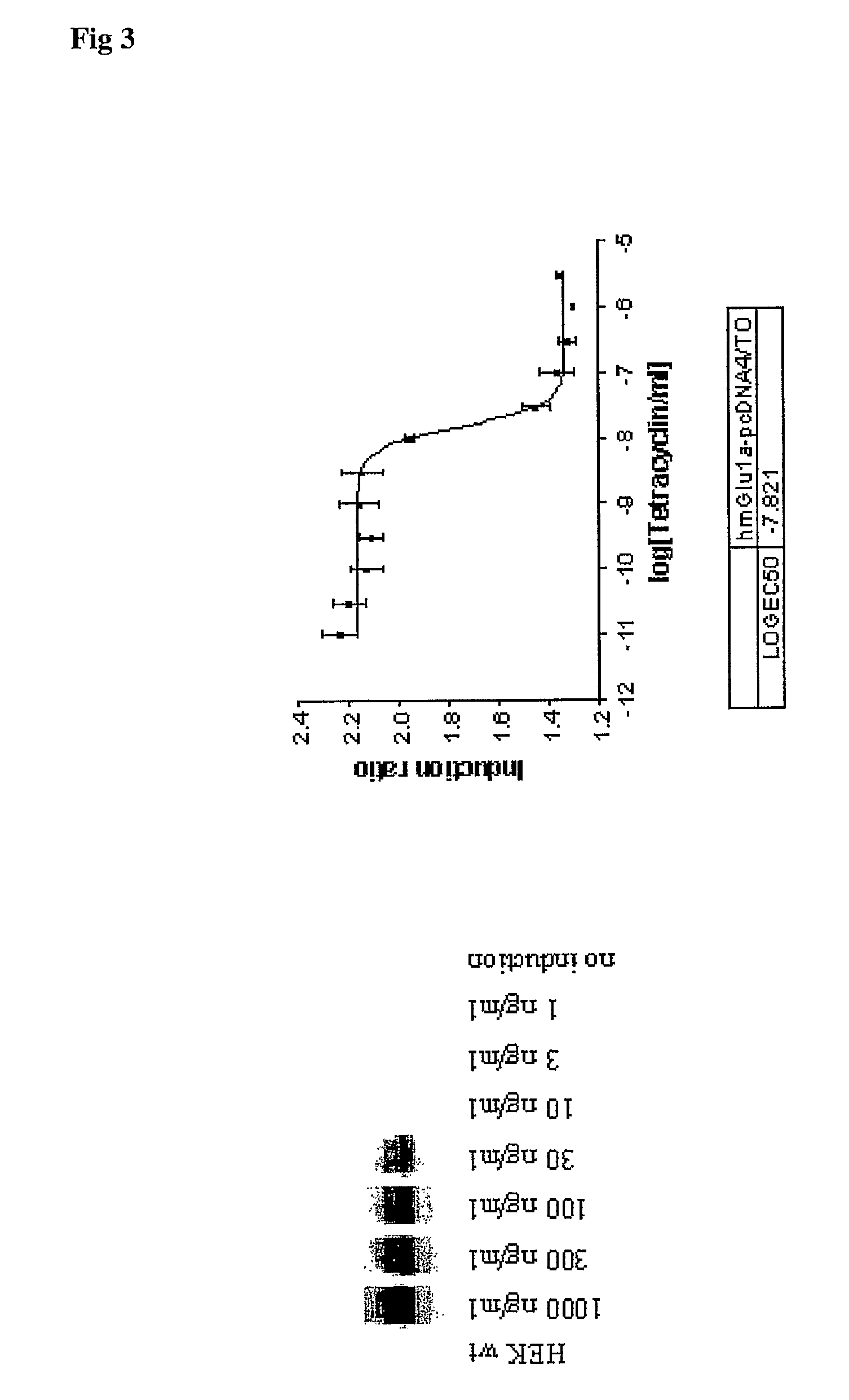
In a first aspect the present invention provides an inducible expression vector encoding a metabotropic glutamate receptor. In particular a tetracycline inducible expression vector such as for example the commercially available pcDNA4 / TO mammalian expression vector (Invitrogen, Carlsbad, Calif. USA) comprising the nucleotide sequence encoding for a member of the Group I mGluRs, in particular for the human mGluR1a (SEQ ID No1) or mGluR5 receptor (SEQ ID No.3). In a more preferred embodiment the inducible expression vector is selected from the tetracycline inducible expression plasmids hmGlu1a-pcDNA4 / TO (FIG. 4) and hmGlu5a-pcDNA4 / TO (FIG. 5). In a second aspect, the present invention provides a cell line comprising any of the aforementioned inducible expression vectors. In particular the T-Rex-293 cells stably transfected with the tetracycline inducible expression plasmids hmGlu1a-pcDNA4 / TO (FIG. 4) and hmGlu5a-pcDNA4 / TO (FIG. 5) which where deposited at the Belgian Coordinated Collections of Microorganisms (BCCM) as T-Rex-293-hmGlu1a-pcDNA4 / TO clone on Jun. 24, 2004. In a third aspect the present invention provides a method to identify compounds capability to modulate the activity of a metabotropic glutamate receptor said method comprising the steps of; contacting the aforementioned cell line with the compound to be tested, and determining the effect of said test compound on the metabotropic glutamate receptor activity. The effect on the metabotropic glutamate receptor activity is typically determined by assessing the change in intracellular calcium, in particular using a fluorescent dye such as for example fluo-3-AM. It is also an object of the present invention to provide a method to identify a compound capable to interact with a metabotrobic glutamate receptor, in particular with a Group I mGluR receptor, said method comprising the steps of contacting the cells according to the invention with the compounds to be tested under appropriate conditions and determining the binding of said test compounds to the cells.
Owner:JANSSEN PHARMA NV
Novel bone mineralization proteins, DNA, vectors, expression systems
InactiveUS20060019392A1Good curative effectEasy assessment processOrganic active ingredientsFungiBone formationPlasmid dna
The present invention is directed to isolated nucleic acid molecules that encode LIM mineralization protein, or LMP. The invention further provides vectors comprising nucleotide sequences that encode LMP, as well as host cells comprising those vectors. Moreover, the present invention relates to methods of inducing bone formation by transfecting osteogenic precursor cells with an isolated nucleic acid molecule comprising a nucleotide sequence encoding LIM mineralization protein. The transfection may occur ex vivo or in vivo by direct injection of virus or naked plasmid DNA. In a particular embodiment, the invention provides a method of fusing a spine by transfecting osteogenic precursor cells with an isolated nucleic acid molecule having a nucleotide sequence encoding LIM mineralization protein, admixing the transfected osteogenic precursor cells with a matrix and contacting the matrix with the spine. Finally, the invention relates to methods for inducing systemic bone formation by stable transfection of host cells with the vectors of the invention.
Owner:EMORY UNIVERSITY
Genetically modified Babesia parasites expressing protective tick antigens and uses thereof
The present invention relates to methods for stable transfection of Babesia parasites, and for vaccines conferring immunity against parasitic arthropods.
Owner:US SEC AGRI +1
Method for extracting polysaccharide from lycium barbarum
InactiveCN107540759AInhibit aggregationTreatment hasOrganic active ingredientsNervous disorderDiseaseChemistry
The invention relates to polysaccharide extracted from lycium barbarum, an extraction method of the polysaccharide and the application of the polysaccharide in preparation of a drug for treating an Alzheimer's disease and specifically relates to a method for extracting polysaccharide LBP1C-2 (lycium barbarum polysaccharide 1C-2) from the lycium barbarum. The method comprises the following steps: extracting enzyme-water combined extraction lycium barbarum crude polysaccharide from the lycium barbarum and purifying the crude polysaccharide, so as to obtain the polysaccharide LBP1C-2; determiningthe polysaccharide LBP1C-2 as pectin through acid hydrolysis, methylation and nuclear magnetic resonance analysis. An in-vitro experiment shows that the polysaccharide LBP1C-2 can inhibit the generation of CHO / APPBACE1 cells of stable transfection APP and BACE1 and A beta 42 in HEK293-APPsw cells formed by mutation of stable transfection APP Switish in a dose-dependent manner, and the pectin caninhibit the aggregation of A beta 42.
Owner:贵州中科健生物医药有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com