Building of stable cell line carrying orthogonal tRNA/arginyl tRNA synthetase
A cell line and enzyme synthesis technology, applied in the field of biopharmaceuticals, can solve problems such as the bottleneck of industrial application of gene codon expansion technology, achieve specific modification and achieve stable expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
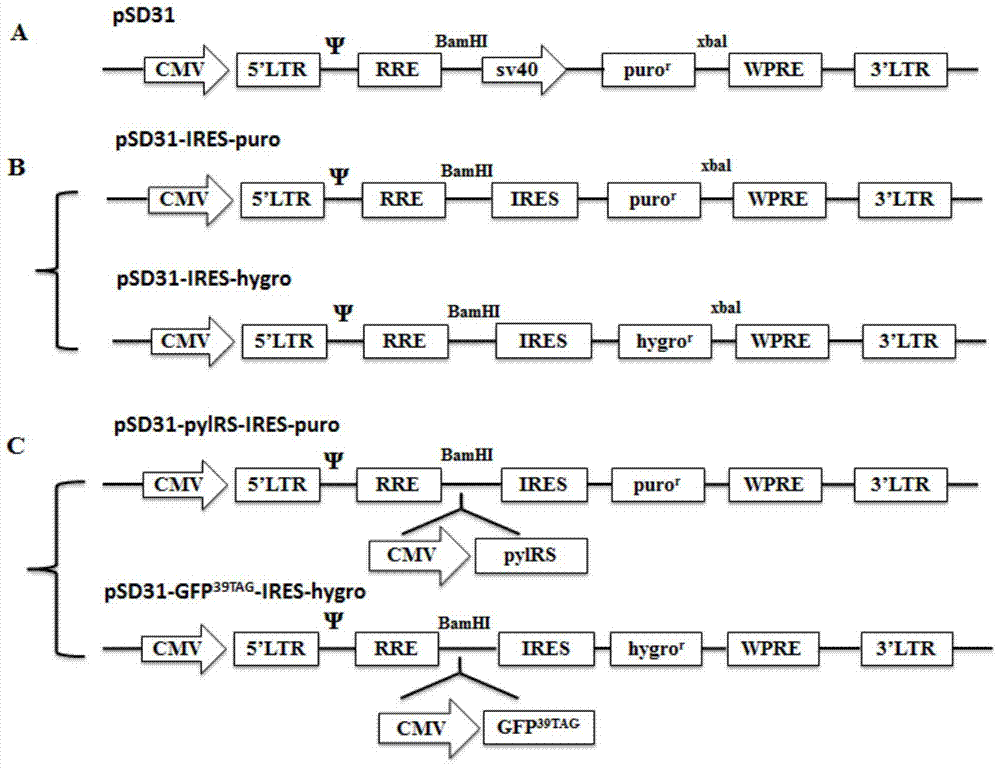
[0061] Example 1: Construction and acquisition of double lentiviral vector
[0062] (1) Obtaining the carrier skeleton
[0063] The backbone of the double lentiviral vector is the lentiviral vector pSD31 (Zhang Jing. et al. RNA, 2007, 13:1375–1383.), in which the sv40 promoter promotes the puromycin resistance gene protein puro R expression.
[0064] (2) Primer design for SOE PCR
[0065] The inventors used SOE PCR to splice the DNA fragments of the internal ribosome entry sequence (IRES) and the puromycin (puromycin) resistance gene / hygromycin B (hygromycin) resistance gene to obtain IRES-puro and IRES- hygro fragment, the specific primers are shown in the table below.
[0066] Table 1: List of SOE PCR primers
[0067] mutation site Sequence (5'-3' direction) IRES-hygro-for (BamHI) CGGGATCCAATTCCGCCCCTCTC IRES-hygro-middle-for: CCCACAAGGAGACGACCTTCCATGAAAAAGCCTGAACTCACC IRES-hygro-middle-rev: GGTGAGTTCAGGCTTTTTCATGGAAGGTCGTCTCCTTGTGGG ...
Embodiment 2
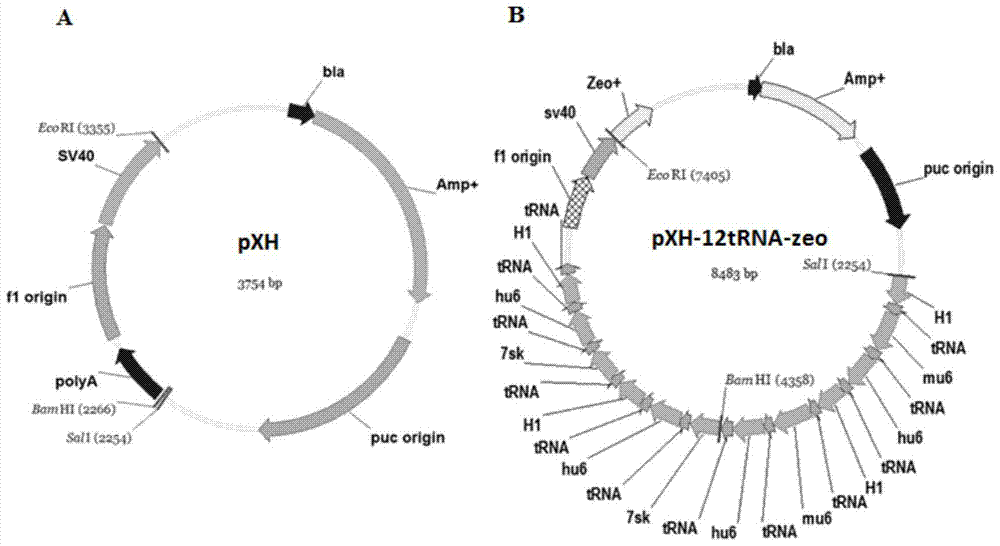
[0070] Example 2: Construction and acquisition of pXH-12tRNA-zeo vector
[0071] In order to ensure the expression of tRNA, it is necessary to clone multiple copies of the promoter-tRNA expressed in tandem into an appropriate vector. In the present invention, the pXH blank vector is used as the backbone, and the zeomycin-polyA sequence is introduced into the back of the SV40 promoter through the EcoRI restriction site, so that it has bleomycin resistance. After that, 12 copies of the promoter-tRNA sequence were cloned into the pXH-zeo vector using the SalI restriction site. In order to avoid the possibility of recombination of repeated sequences, 4 different tRNA promoters were used: 7sk / hu6 / H1 / mu6. Finally, the vector bjmu-12t-zeo for screening tRNA was obtained.
[0072] (1) Obtaining the carrier skeleton
[0073] The backbone of the pXH-12tRNA-zeo vector is the vector pXH, which is a shuttle vector transformed from the PUC19 vector, which has the advantages of being abl...
Embodiment 3
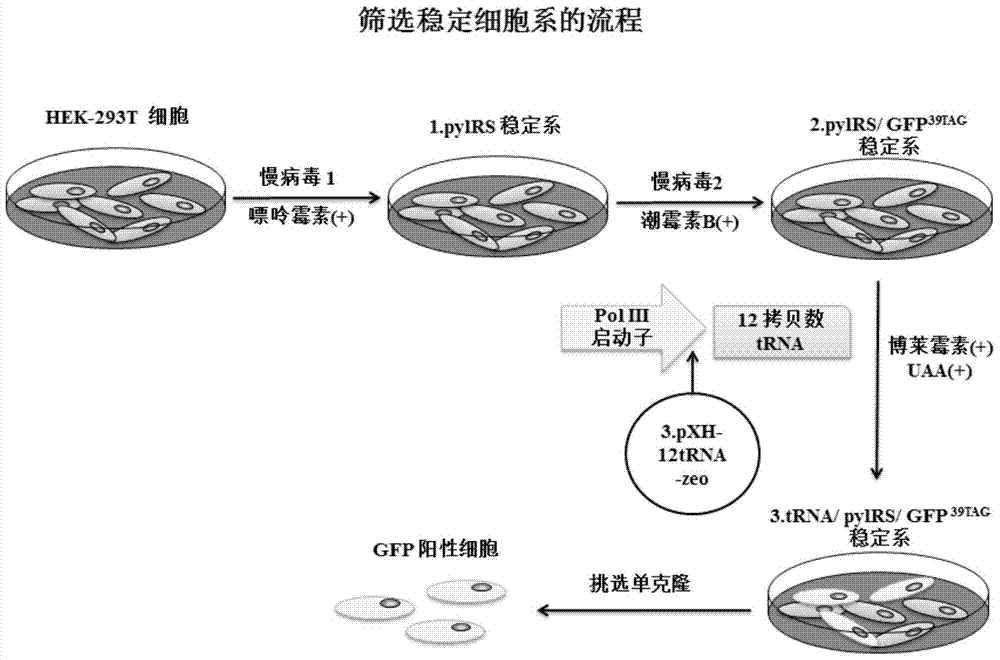
[0083] Example 3: Screening of Stable Cell Lines
[0084] (1) Packaging and transduction of lentivirus, including the following steps:
[0085] a.HEK 293T cell plating: use medium A, components (DMEM+10%FBS,1×NEAA,without sodium pyruvate), cell digestion and counting, the number of cells seeded in each well of a six-well plate is 4×10 5 cells / well.
[0086] b. Lentivirus packaging: Transfection is performed at a cell density of 70% to 80%, and the mixture of plasmids and transfection reagents is shown in Table 3-1. Six hours after transfection, medium B (DMEM+3% FBS, 1×NEAA, With Sodium Pyruvate) was changed. Continue to cultivate. The virus liquid was collected 48 hours and 72 hours after transfection, and filtered with a PVDF membrane syringe filter with a pore size of 0.45 μm.
[0087] Table 3-1. Plasmid ratio for lentiviral packaging
[0088] Plasmids / Transfection Reagents Dosage per well Opti-MEM 200μl transfer vector 0.72μg pRSV 0.64...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com