Method for suspension culture of sensitive cells and method for producing blue ear disease vaccine by using sensitive cells
A suspension culture, cell technology, applied in the field of medicine and biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
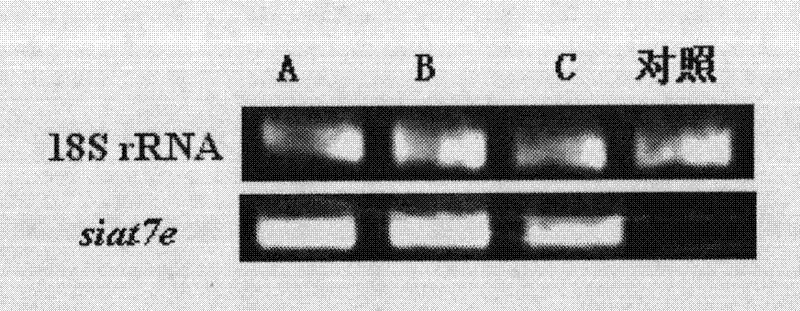
[0064] Embodiment 1 Utilizes genetic modification suspension culture Marc145 cell
[0065] 1) Stable transfection of Marc145 cells with an expression vector containing the siat7e gene;
[0066] (1) Escherichia coli DH5α competent cells were transfected with a full-length human siat7e gene expression vector (Cat.No.EX-V1581-M03, Genecopoeia). Plasmid DNA was extracted using an endotoxin-free plasmid extraction kit (QIAGEN).
[0067] (2) Day 1: transfer 3×10 5 Marc145 cells (provided by the China Veterinary Drug Administration) were incubated in a 6-well plate for 22 hours with a confluence of about 70%.
[0068] (3) On the second day, 5 μg of plasmid DNA was diluted in 250 μL Opti-MEM medium, and mixed; 15 μL liposome was diluted in 250 μL Opti-MEM medium, mixed gently, and allowed to stand for 10 minutes.
[0069] (4) Mix the solutions prepared above, and let stand at room temperature for 20min; replace each well of the 6-well plate described in step (2) with 2 mL of Opti-M...
Embodiment 2-4
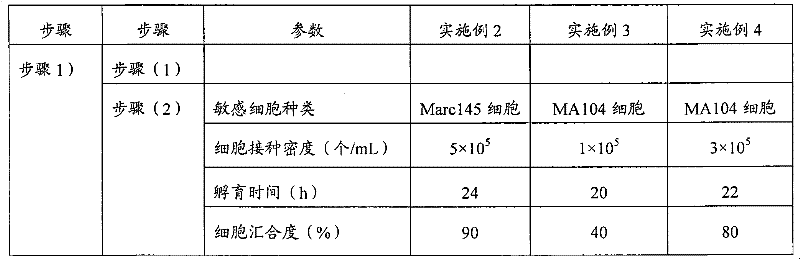
[0082] Except that the experimental conditions were changed according to Table 1, the same operations as in Example 1 were carried out.
[0083] Table 1
[0084]
[0085]
Embodiment 5
[0086] Embodiment 5 utilizes the method for producing PRRS vaccine by the Marc145 cell of large-scale culture genetic modification suspension culture
[0087]A part of the cells verified by suspension culture (Marc145 cells, Example 1) was frozen and stored, and the other part was continued to be cultured in the cell culture flask. When the cells grow normally in cell culture flasks and their viability is greater than 90%, they are inoculated into shake flasks. Expand cultured cells in shake flasks, press 2×10 5 The cells / mL cell density were inoculated into a 120L bioreactor (Beijing Qingda Tianyi Technology Co., Ltd.), and the cell culture conditions were as follows:
[0088] Working volume: 60L
[0089] Cell culture temperature: 37°C
[0090] pH: 7.2
[0091] Dissolved oxygen: 50%;
[0092] Cell culture medium: Marc145 bioreactor high-density cell culture medium (Cat No.MD900, Beijing Qingda Tianyi Technology Co., Ltd.)
[0093] Cultured to a cell density of 2×10 6 C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com