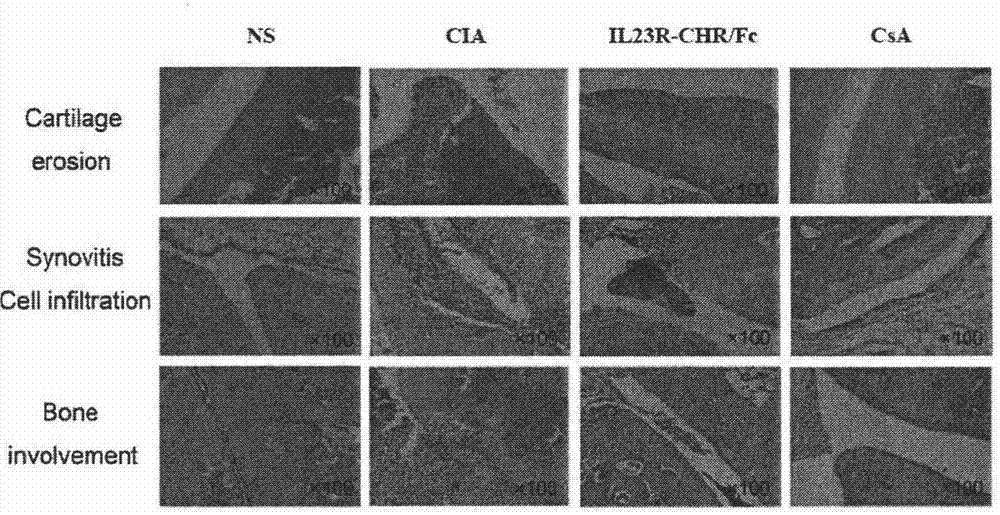
Novel IL23 antagonist
An IL-23, fusion protein technology, applied in allergic diseases, peptide/protein components, drug combinations, etc., can solve problems such as poor stability, difficult endotoxin removal, and short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Example 1: Construction of pcDNA3.1(+)-IL23R-CHR / Fc expression vector
[0093] 1) Construction of IL23R-CHR / Fc fusion gene by Overlap PCR
[0094] a) Using the human leukocyte cDNA library or existing related plasmids as templates, amplify the signal peptide (SP, signal peptide) with two pairs of upstream and downstream primers, F(SP) and R(SP), F(CHR) and R(CHR), respectively. ) and IL23R-CHR gene; mRNA was extracted from normal human peripheral blood leukocytes, reverse-transcribed to obtain the first strand of cDNA, and then used as a template to amplify IgG1Fc gene with F (Fc) and R (Fc) as upstream and downstream primers . Using high-fidelity enzyme amplification, Touch-down PCR conditions enhance the efficiency of specific band amplification. Signal peptide, IL23R-CHR and Fc amplification products were separated and identified by agarose gel electrophoresis, and about 80bp, 570bp and 700bp fragments were recovered, respectively.
[0095] Table 1 Primer sequence...
Embodiment 2
[0107] Example 2: Transient transfection expression of rhIL-23R-CHR / Fc protein
[0108] 1) About 24 hours before transfection, subculture the 293T cells again, try to ensure that the cells are in good condition and the density reaches 70-90% at the time of transfection; before transfection, replace the old medium with fresh DMEM culture without serum base.
[0109] 2) According to the different culture devices, prepare a sufficient amount of transfection complex according to the ratio of PEI(ug):plasmid (ug)=2:1 to 5:1; the plasmid and PEI are diluted in an equal volume of opti-MEM respectively, Then add the PEI dilution to the DNA dilution, mix gently, and incubate at room temperature for 10-15 minutes to obtain a PEI / DNA transfection complex of 1 / 10 times the volume of the medium.
[0110] 3) Evenly drop the transfection complex into the culture device, shake gently to mix it with fresh medium, replace with serum-free medium after 4-6 hours of transfection, culture for 3-5 ...
Embodiment 3
[0111] Example 3: Screening of stable expression cell lines of rhIL-23R-CHR / Fc protein
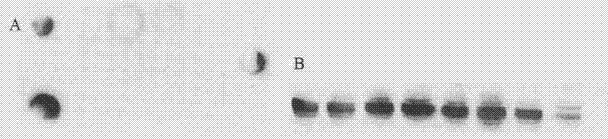
[0112] Take CHO cells in the logarithmic growth phase at an appropriate concentration and add them to the electric shock cup, then add an appropriate amount of linearized expression plasmids, mix and let stand on ice for about 15 minutes, and set the corresponding parameters of the electroporation instrument to complete. Transfer to FBS-containing DMEM-F12 medium for culture (liposomes can also be used to transfect the plasmid), and when the cell growth state is restored, positive cells are screened with 1 mg / ml G418. Observe the state of the cells, change the medium every 3 days, and after 10 to 14 hours of culture, use the limiting dilution method to screen high-expression monoclonal cell lines using a 96-well culture plate. Immunospot method and Western blot can be used to compare the expression levels of different clones for screening ( Figure 4 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com