Cephalosporin C acylase mutant as well as preparation method and application thereof
A cephalosporin and acylase technology, applied in the field of bioengineering, can solve the problems of low enzyme activity and low industrial production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
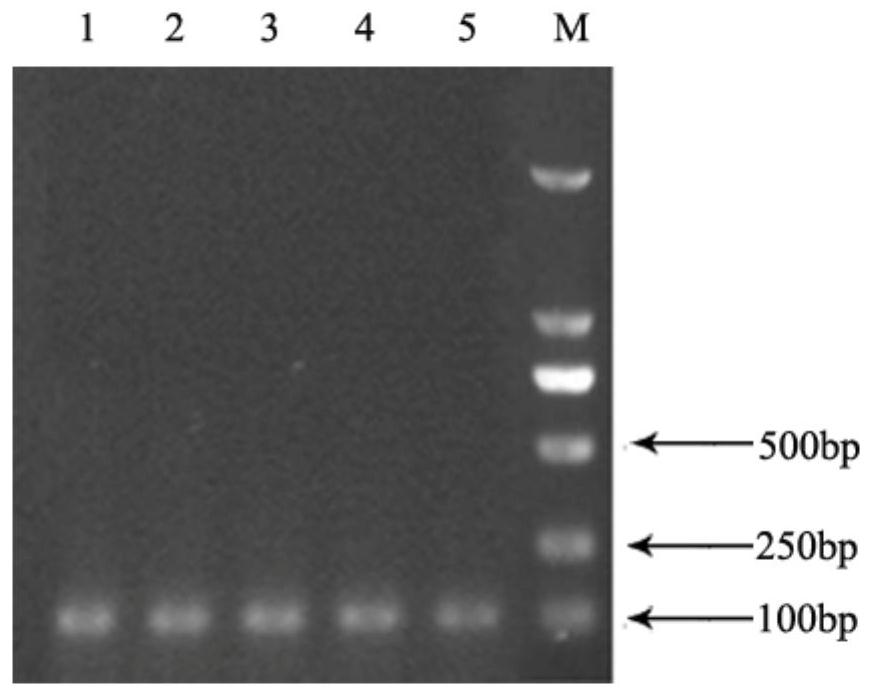
[0034] (1) The gene of interest was cloned using SEQ ID NO: 4, the wild-type gene of CPC acylase, as a template. The primer sequences were: upstream primer F1: GGGAATTCCATATGCT F2: GAGAGTTCTGCACCG downstream primer R1: CCCGGAATTCTCATGG R2: CTTGAAGTTGAAGTTGAAGG, wherein F1 and R2 contained 20bp identical Homology arms, F2 and R1 contain 20bp homology arms, so as to carry out double point mutations.
[0035] (2) The PCR amplification system is as follows:
[0036]
[0037] The PCR reaction conditions were pre-denaturation at 98°C for 3 min; denaturation at 98°C for 10 s, annealing at 45°C for 5 s, extension at 72°C for 3 min, and 30 cycles; extension at 72°C for 10 min.
[0038] (3) Subsequent purification of the PCR product was performed using a purification kit, and the obtained target gene was double-digested with BamHI and HinDIII endonucleases. For the plasmid vector PET-28a + Carry out the same double digestion to obtain the recombinant plasmid PET-28a + -CPCA. Esche...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com