Cephalosporin C acylase mutant with one or any point mutations and preparation method thereof
A technology of acylase and mutants, which is applied in the field of drug production and can solve problems such as low enzyme activity, high impurities, and poor thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]Example 1 Construction of CPC Acylase Mutants
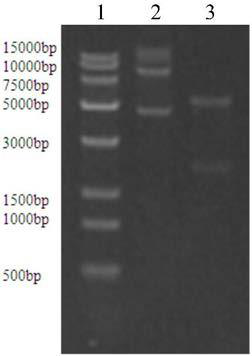
[0026] 1.1 Cloning of CPC acylase gene
[0027] The CPC acylase gene derived from Pseudomonas sp.130 (see SEQ ID NO.1, synthesized by GenScript) was synthesized from the whole gene, and the gene was cloned into the expression vector pUC57 to obtain the recombinant plasmid pUC57-CPCA. Use pUC57-CPCA as a template to amplify the target gene fragment. The DNA polymerase PrimeSTAR used in PCR and the corresponding buffer and dNTP solution were all purchased from Treasure Biotechnology Company. The primer sequence was synthesized by GenScript, and the primer sequence is: F: GGGAATTC CATATG CTGAGAGTTCTGCACCG (the underlined base is the NdeI recognition site) R: CCG GAATTC TCATGGCTTGAAGTTGAAGGG (underlined bases are EcoRI recognition sites)
[0028] PCR amplification reaction system: Sterilized water: 32.5 μL
[0029] 5×PrimeSTAR buffer (Mg 2+ Plus): 10 μL
[0030] dNTP solution (2.5mM each): 4μL
[0031] DNA polymerase ...
Embodiment 2
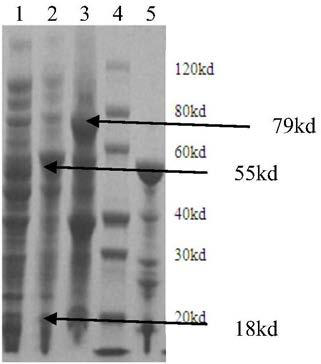
[0053] Embodiment 2 conversion reaction
[0054] 2.1 Transformation reactions catalyzed by mutant 1
[0055] Reaction conditions: CPC 1g / L, input enzyme activity 600-800U / L (immobilized enzyme or liquid enzyme), reaction temperature 25°C, result: reaction time 40-120min, conversion rate 98-99%.
[0056] 2.2 Transformation reactions catalyzed by mutant 2
[0057]Reaction conditions: CPC 1g / L, input enzyme activity 600-800U / L (immobilized enzyme or liquid enzyme), reaction temperature 14-25°C, result: reaction time 20-120min, conversion rate over 99%.
[0058] 2.3 Transformation reactions catalyzed by mutant 3
[0059] Reaction conditions: CPC 1g / L, input enzyme activity 600-800U / L (immobilized enzyme or liquid enzyme), reaction temperature 14-25°C, result: reaction time 20-120min, conversion rate over 99%.
[0060] 2.4 Transformation reactions catalyzed by mutant 4
[0061] Reaction conditions: CPC 1-5g / L, input enzyme activity 600-4000U / L (immobilized enzyme or liquid enzy...
Embodiment 3
[0072] Embodiment 3 impurity detection
[0073] Chromatographic column: phenomenex (Philomen) filler: Gemini 5um C18;
[0074] Mobile phase preparation:
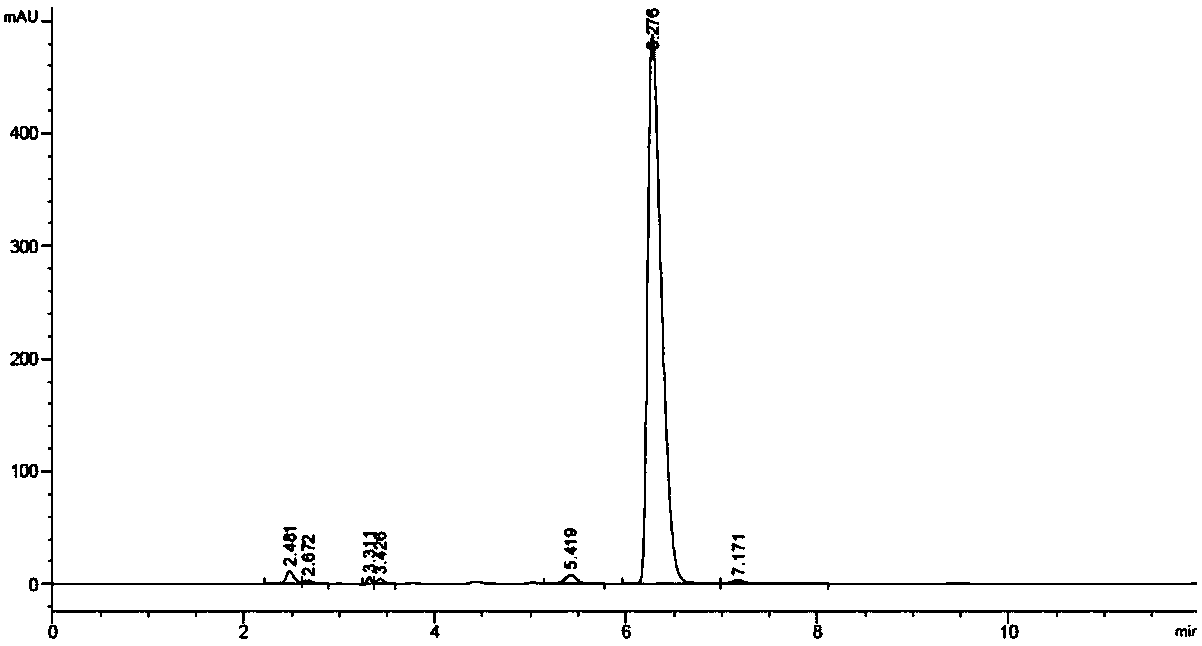
[0075] Buffer: 1.54g NH 4 COOCH 3 →1000mL pH=6.0; buffer: acetonitrile=95:5 (1000mL buffer added 53mL acetonitrile); detection wavelength: 260nm column oven temperature: 25°C; flow rate: 1.2mL / min. Respectively D-CPC, D-7ACA, DO-CPC, DO-7ACA, CPC, 7-ACA in chronological order, using mutant 1-6 to react, impurity analysis is less than 0.3% ( image 3 , Mutant 6 reaction result), comparative example S12 impurity analysis is greater than 0.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com