Mutant cephalosporin C acylase, method for preparing same and method for converting 7-aminocephalosporin acid (ACA)
A cephalosporin and acylase technology, which is applied in the field of bioengineering, can solve the problems of low catalytic activity, large external influence on enzyme activity, product inhibition, etc., and achieves the effect of fast conversion rate, small external influence and small inhibition.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
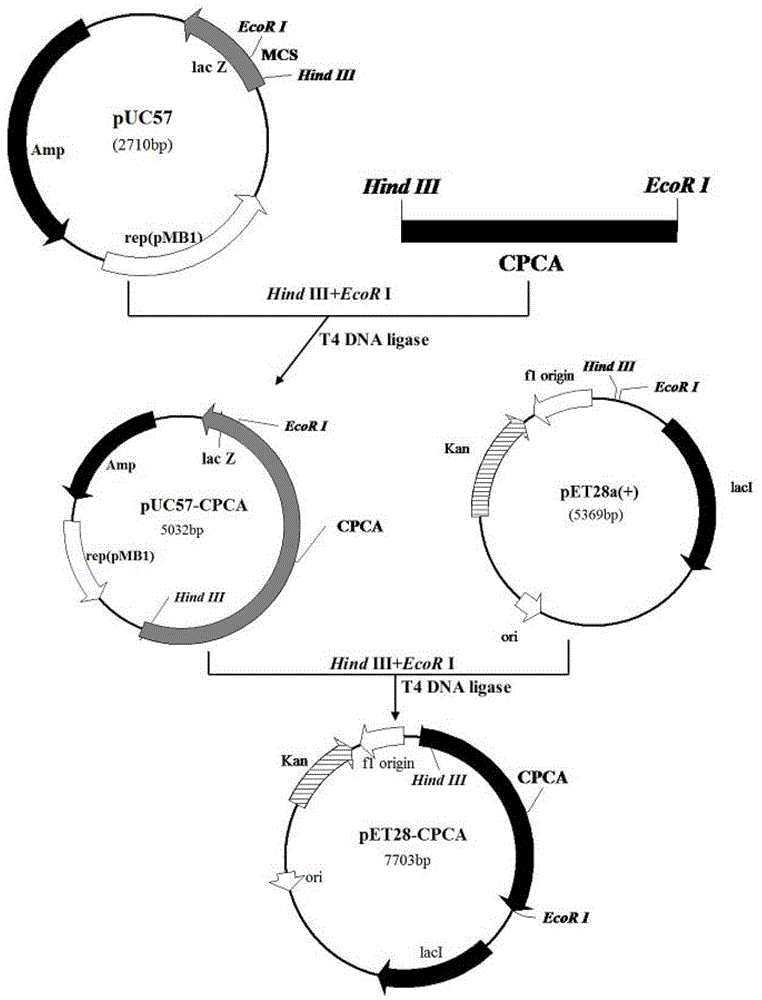
[0052] Another aspect of the present invention also provides a kind of preparation method of aforementioned mutant cephalosporin C acylase, comprises the following steps:
[0053] a) Perform site-directed mutation, site-directed saturation mutation or multi-point mutation on the gene sequence having SEQ ID NO.2 in the sequence listing to obtain the gene sequence having SEQ ID NO.4 in the sequence listing; having SEQ ID NO.6 in the sequence listing The gene sequence; it has the gene sequence of SEQ ID NO.8 in the sequence table; under stringent conditions, it can hybridize with the gene sequence defined by any one of a, b or c and encode a protein with cephalosporin C acylase activity Gene sequence; a mutant gene that has more than 90% homology with the gene sequence defined in any one of a, b or c and encodes a gene sequence of a protein with cephalosporin C acylase activity;
[0054] b) inserting the mutant gene into the plasmid to obtain an expression vector;
[0055] c) tr...
Embodiment 1
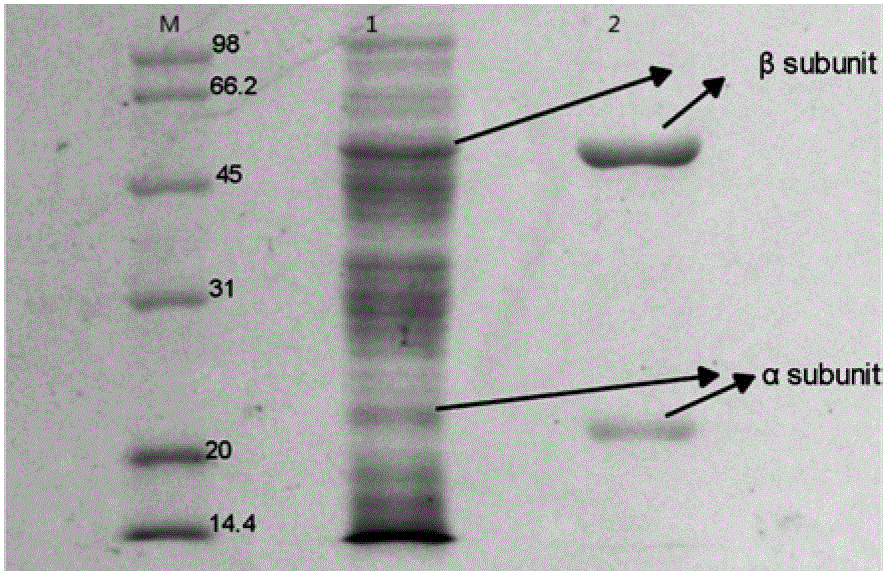
[0092] a) Using the plasmid extracted from the Top10' strain containing the wild-type cephalosporin C acylase expression vector pET28-CPCA clone strain as the mutation template, the extracted plasmid has the gene sequence of SEQ ID NO.2 in the sequence table. Design mutation primers according to the gene sequence of the extracted plasmid, perform site-directed mutation at position 288 by reverse PCR in the reverse PCR reaction system, first pre-denature at 95°C for 5 minutes, then denature at 94°C for 30S, and anneal at 60°C 1 min, extended at 72°C for 8 min, repeated denaturation, annealing, and extension 18 times, and finally extended at 72°C for 10 min to obtain the PCR product. The preparation method of the reverse PCR reaction system is to mix 5 μl of 10*pfxbuffer, 1 μl of forward primer, 1 μl of reverse primer, and 1 μl of Mg 2+ , 1 μl of template DNA, 10 μl of 5*Enhancer Buffer, 2 μl of dNTPs, 0.5 μl of pfx high-fidelity DNA polymerase with an enzyme activity of 2.5U / μl...
Embodiment 2
[0099] a) Using the plasmid extracted from the Top10' strain containing the wild-type cephalosporin C acylase expression vector pET28-CPCA I clone strain as the mutation template, the extracted plasmid has the gene sequence of SEQ ID NO.2 in the sequence table. Design mutation primers according to the gene sequence of the extracted plasmid, perform reverse PCR reaction in the reverse PCR reaction system, and perform site-directed mutation at 417 again based on the mutation at 288. First, pre-denature at 95°C for 5 minutes, and then at 94°C Denature for 30S, anneal at 60°C for 1 min, extend at 72°C for 8 min, repeat denaturation, annealing, and extension 18 times, and finally extend at 72°C for 10 min to obtain the PCR product. The preparation method of the reverse PCR reaction system is to mix 5 μl of 10*pfx buffer, 1 μl of forward primer, 1 μl of reverse primer, 1 μl of Mg2+, 1 μl of template DNA, 10 μl of 5*Enhancer Buffer, 2 μl of dNTPs, 0.5 μl of pfx high-fidelity DNA poly...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com