Synthesis method of high-purity 7-amino-3-propylene-1-yl-3-cephem-4-carboxylic acid
A technology of aminocephalosporanic acid and synthesis method, which is applied in the field of chemical synthesis, can solve problems such as harsh operating conditions, poor product purity, and difficult recycling of waste liquid, and achieve the effects of easy recycling, less reagents, safe and environmentally friendly recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
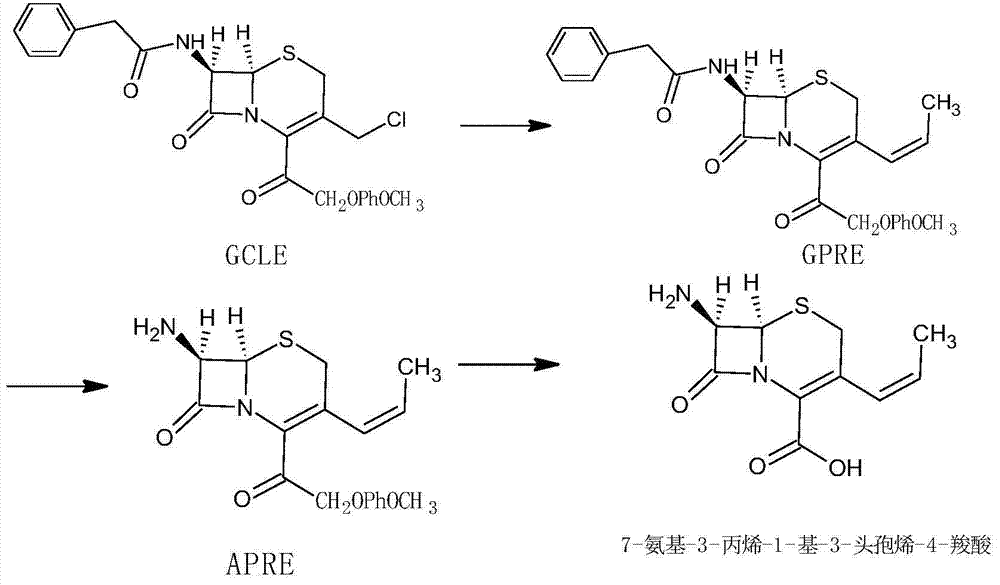
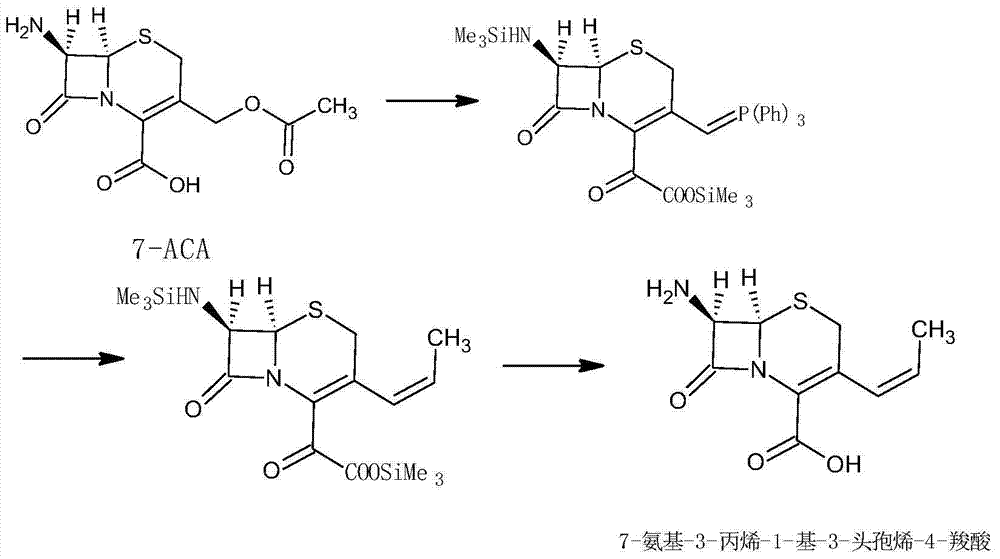
Image
Examples
Embodiment 1
[0037] (1) Preparation of formula (I) compound:
[0038] Water 100mL, cool down to 0-10°C, add 20g of deacetyl-7-aminocephalosporanic acid under stirring, add dropwise 10% sodium bicarbonate solution, stir until clear, add 4.5g of sodium bromide, 0.9g of 2, To 2,6,6-tetramethylpiperidine nitrogen oxide (TEMPO), 66 ml of 10.2% sodium hypochlorite solution was added dropwise, reacted for 2 hours, and 9% hydrochloric acid solution was added to adjust the pH to 3.2 to obtain 18.4 g of the product;
[0039] (2) Preparation of 7-amino-3-propene-1-yl-3-cephem-4-carboxylic acid
[0040] Put 15g of the compound of formula (I) into 60mL of dichloromethane, add 16.6mL of hexamethyldisilazane under stirring, reflux for 8 hours, cool down to 0-5°C, add 27.1g of ethyltriphenylphosphine bromide, After reacting for 2 hours, 180 mL of methanol was added for crystallization to obtain 14.4 g of the product. The total molar yield is 84.5%, the purity is 99.6%, and the trans isomer accounts for ...
Embodiment 2
[0042] (1) Preparation of formula (I) compound:
[0043] Water 80mL, cool down to 0-10°C, add 20g of deacetyl-7-aminocephalosporanic acid under stirring, add dropwise 5% sodium hydroxide solution, stir until clear, add 2g of potassium bromide, 0.5g 2,2 , 6,6-tetramethylpiperidine nitrogen oxide (TEMPO), 69 mL of 10.2% sodium hypochlorite solution was added dropwise, reacted for 3 hours, and 40% sulfuric acid solution was added to adjust the pH to 3.5 to obtain 18.2 g of the product;
[0044] (2) Preparation of 7-amino-3-propene-1-yl-3-cephem-4-carboxylic acid
[0045] Put 15g of the compound of formula (I) into 80mL of dichloromethane, add 32mL of N,O-bistrimethylsilylacetamide under stirring, react at room temperature for 3 hours, cool down to 0-5°C, add 27.3g of ethyltriphenyl Phosphine bromide was reacted for 3 hours, and 200 mL of ethanol was added for crystallization to obtain 14.6 g of the product. The total molar yield is 84.8%, the purity is 99.7%, and the trans isomer...
Embodiment 3
[0047] (1) Preparation of formula (I) compound:
[0048] Water 120mL, cool down to 0-10°C, add 20g of deacetyl-7-aminocephalosporanic acid under stirring, add dropwise 8% sodium carbonate solution, stir until clear, add 1g of sodium bromide, 0.7g2,2,6 , 6-tetramethylpiperidine nitrogen oxide (TEMPO), 80mL of calcium hypochlorite solution with a mass fraction of 8% was added dropwise, and after reacting for 2.5 hours, a phosphoric acid solution with a mass fraction of 85% was added to adjust the pH to 3.5 to obtain 18g of the product;
[0049] (2) Preparation of 7-amino-3-propen-1-yl-3-cephem-4-carboxylic acid
[0050] Put 15g of the compound of formula (I) into 100mL of dichloromethane, add 25mL of trimethylchlorosilane under stirring, reflux for 7 hours, cool down to 0-5°C, add 27.5g of ethyltriphenylphosphine bromide, and react for 3 hours , 150 mL of isopropanol was added for crystallization to obtain 14.2 g of the product. The total molar yield is 81.6%, the purity is 99...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com