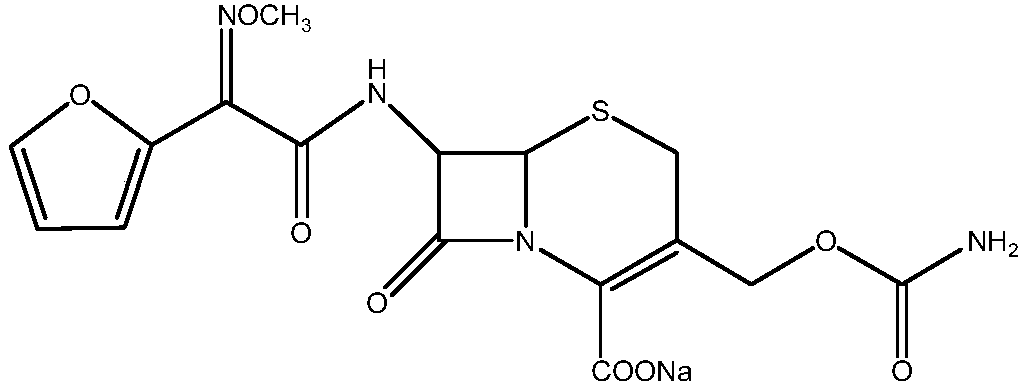
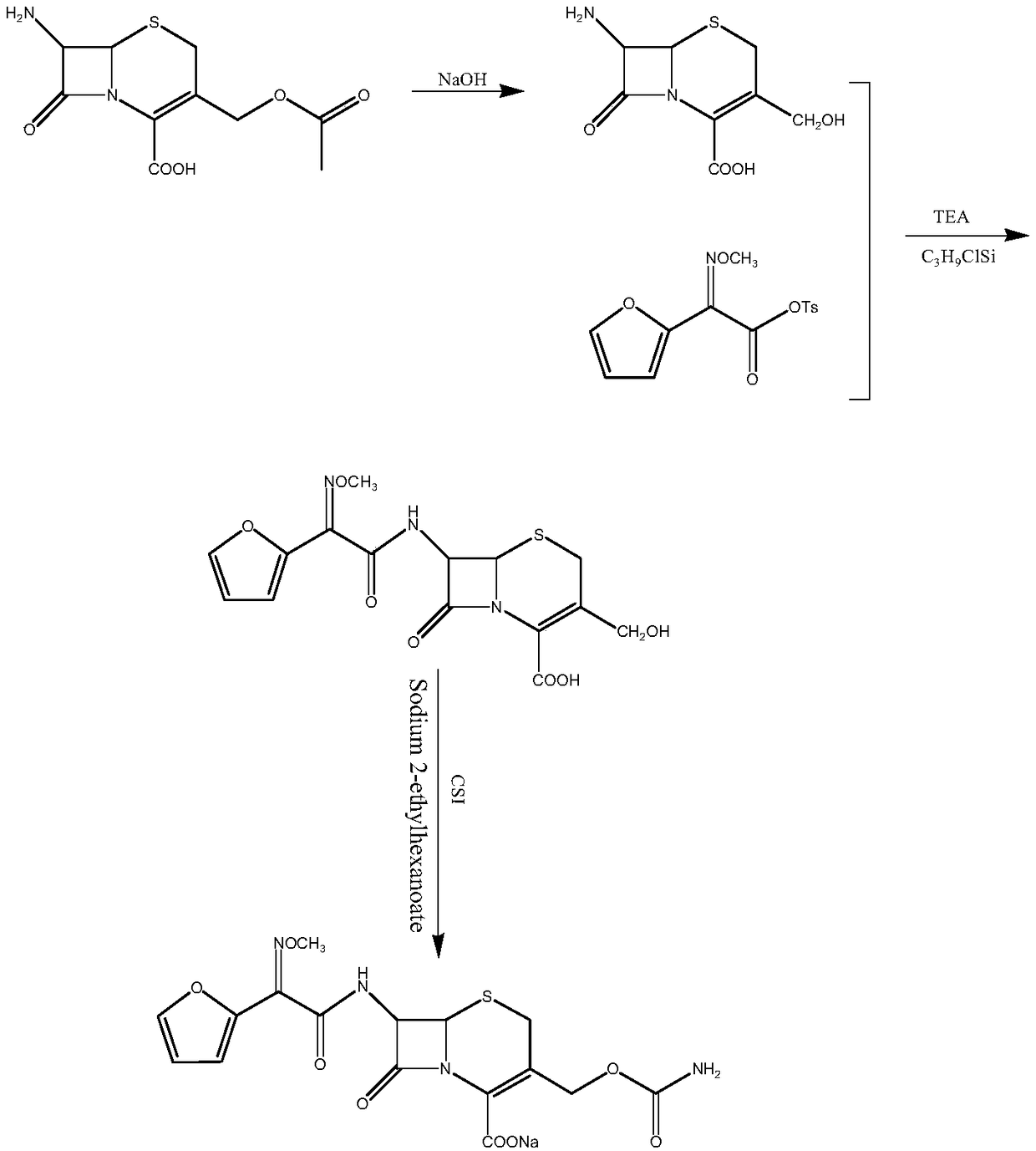
Cefuroxime sodium synthesizing method
A technology of cefuroxime sodium and a synthesis method, which is applied in the field of medicine and chemical industry, can solve the problems of easily polluted environment, complicated operation, many side reactions, etc., and achieves the effects of easy availability of raw materials, simple operation and reduction of operation steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A kind of synthetic method of cefuroxime sodium, comprises the steps:
[0030] (1) Dissolve 21.76g (0.08mol) of 7-ACA in 100ml of water in an ice bath at 0-5°C, add dropwise 1mol / L sodium hydroxide solution, adjust the pH to 8.5, and carry out the hydrolysis reaction 2.5 h, to obtain a reaction solution containing D-7-ACA, continue to maintain the temperature at 0-5 ° C, add triethylamine (24.28g, 0.24mol) and trimethylchlorosilane (8.69g, 0.08mol), dropwise add SMIF-Ts Chloroform solution (SMIF-Ts0.096mol, 30ml of chloroform), keep warm for 2.5h, add 20ml of distilled water, separate layers and collect the water phase, adjust the pH to 2.5, crystallize for 2.5h, filter, wash with water until neutral, vacuum at 50°C Dry to obtain DCCF (20.40 g, 80.7%); 1 HNMR(d 6 -DMSO) δ: 9.75 (d, 1H), 7.83 (bs, 1H), 6.69 (m, 2H), 5.60 (s, 1H), 5.02 (d, 1H), 4.73 (m, 2H), 3.90 (s , H), 3.52-3.32 (m, 2H);
[0031] (2) Dissolve 15.80 g of the DCCF in 120 ml of methyl acetate, lower t...
Embodiment 2
[0033] A kind of synthetic method of cefuroxime sodium, comprises the steps:
[0034] (1) Dissolve 21.76g (0.08mol) of 7-ACA in 100ml of water in an ice bath at 0-5°C, add dropwise 1mol / L sodium hydroxide solution, adjust the pH to 9, and carry out the hydrolysis reaction for 2 hours , to obtain a reaction solution containing D-7-ACA, continue to maintain the temperature at 0-5 ° C, add triethylamine (24.28g, 0.24mol) and trimethylchlorosilane (8.69g, 0.08mol), dropwise add SMIF-Ts chloroform Solution (SMIF-Ts0.096mol, 30ml of chloroform), keep warm for 2.5h, add 20ml of distilled water, separate layers and collect the water phase, adjust the pH to 2.3, crystallize for 2h, filter, wash with water until neutral, and dry under vacuum at 50°C. DCCF was obtained (20.96 g, 82.9%);
[0035] (2) Dissolve 15.80 g of the DCCF in 120 ml of methyl acetate, lower the temperature to -10 °C, add CSI (10.61 g, 0.075 mol) dropwise, keep the reaction for 1.0 h, add 30 ml of distilled water, rai...
Embodiment 3
[0037] A kind of synthetic method of cefuroxime sodium, comprises the steps:
[0038] (1) Dissolve 21.77g (0.08mol) of 7-ACA in 100ml of water in an ice bath at 0-5°C, add dropwise 1mol / L sodium hydroxide solution, adjust the pH to 9.5, and carry out the hydrolysis reaction 1.5 h, to obtain a reaction solution containing D-7-ACA, continue to maintain the temperature at 0-5 ° C, add triethylamine (24.28g, 0.24mol) and trimethylchlorosilane (8.69g, 0.08mol), dropwise add SMIF-Ts Chloroform solution (SMIF-Ts0.104mol, chloroform 30ml), keep warm for 2h, add 20ml of distilled water, separate layers and collect the water phase, adjust the pH to 2.0, crystallize for 1.5h, filter, wash with water until neutral, and vacuum dry at 50°C , to obtain DCCF (20.63g, 81.6%);
[0039] (2) Dissolve 15.80g (0.05mol) of the DCCF in 120ml methyl acetate, cool down to -8°C, add CSI (11.32g, 0.08mol) dropwise, keep the reaction for 1.2h, add 30ml distilled water, and heat up to 32°C , reacted for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com