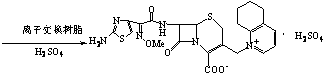
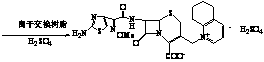
Method for preparing cefquinome sulfate
A technology for cefquinoxime sulfate and cefquinoxime, which is applied in the field of chemical synthesis, can solve the problems of high synthesis cost, difficult handling, large amount of raw materials, etc., and achieves the effects of simple operation, reduced production cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Preparation of Intermediate A
[0032] Add 13.6g of 7-aminocephalosporanic acid and 12.8g of triethylamine into 50mL of acetone, stir and react at 45°C for 3 hours, cool to room temperature, filter, wash with 100mL of deionized water three times, and dry in vacuo. Add 13.6 g of solids obtained by vacuum drying to 50 mL of acetone, add 21.0 g of 2-methoxyimino-2-(2-amino-4-thiazolyl)-(z)-thiocarbazone, add 4.1 g Triethylamine was stirred and reacted at 45°C for 2 hours. After the reaction was completed, 30 mL of deionized water was added, and the pH was adjusted to 1-2 with 2 mol / L sulfuric acid solution. Solid intermediate A was precipitated, filtered, washed, and vacuum-dried.
[0033] (2) Preparation of intermediate B (cefaquine hydroiodide)
[0034] Add the solid obtained in the above reaction step (1) to 50 mL of acetonitrile, add 12.5 g of potassium iodide and 1 mL of 95% phosphoric acid, react under reflux for 2 hours, filter, wash, and dry in vacuum to obtai...
Embodiment 2
[0038] (1) Preparation of Intermediate A
[0039] Add 13.6g of 7-aminocephalosporanic acid and 35.7g of ammonia water (35%) into 50mL of acetone, stir and react at 45°C for 3 hours, cool to room temperature, filter, wash with 100mL of deionized water three times, and dry in vacuo. Add 13.6g of light yellow solid obtained by vacuum drying into 50mL of acetone, add 21.0g of 2-methoxyimino-2-(2-amino-4-thiazolyl)-(z)-thiazolylthioate, Add 4.1 g of ammonia water, stir and react at 45°C for 2 hours, add 30 mL of deionized water after the reaction is complete, adjust the pH to 1-2 with 6 mol / L sulfuric acid solution, precipitate solid A, filter, wash, and vacuum dry.
[0040](2) Preparation of intermediate B (cefaquine hydroiodide)
[0041] Add the solid obtained in the above step (1) into 50 mL of acetonitrile, add 12.5 g of potassium iodide and 1 mL of 95% phosphoric acid, react under reflux for 2 hours, filter, wash, and vacuum dry. Add the obtained light yellow solid into 50mL...
Embodiment 3
[0045] (1) Preparation of Intermediate A
[0046] Add 13.6g of 7-aminocephalosporanic acid and 10.6g of sodium carbonate into 50mL of dichloromethane, stir and react at 50°C for 3 hours, cool to room temperature, filter, wash with 100mL of deionized water three times, and dry in vacuo. Add 13.7g of light yellow solid obtained by vacuum drying into 50mL of acetone, add 21.0g of 2-methoxyimino-2-(2-amino-4-thiazolyl)-(z)-dicarbazolylthioacetate, Add 12.8 g of triethylamine, stir and react at 45°C for 2 hours, add 30 mL of deionized water after the reaction, adjust the pH to 1-2 with 6 mol / L sulfuric acid solution, precipitate solid A, filter, wash, and vacuum dry.
[0047] (2) Preparation of intermediate B (cefaquine hydroiodide)
[0048] Add the solid obtained in the above step (1) into 50 mL of acetonitrile, add 11.8 g of sodium iodide and 1 mL of 95% phosphoric acid, react under reflux for 3 hours, filter, wash, and vacuum dry. Add the obtained light yellow solid into 50mL ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com