Cefuroxime sodium and preparation method thereof
A technology for cefuroxime sodium and cefuroxime acid, which is applied in the field of drug synthesis, can solve problems such as low product yield, and achieve the effects of easy availability of raw materials, simple operation and reduced impurity content
Active Publication Date: 2010-08-25
LIVZON PHARM GRP INC
View PDF1 Cites 21 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
In this synthetic method, the generated intermediate product cefuroxime acid is directly salified without treatment, resulting in a low product yield, only 85%.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
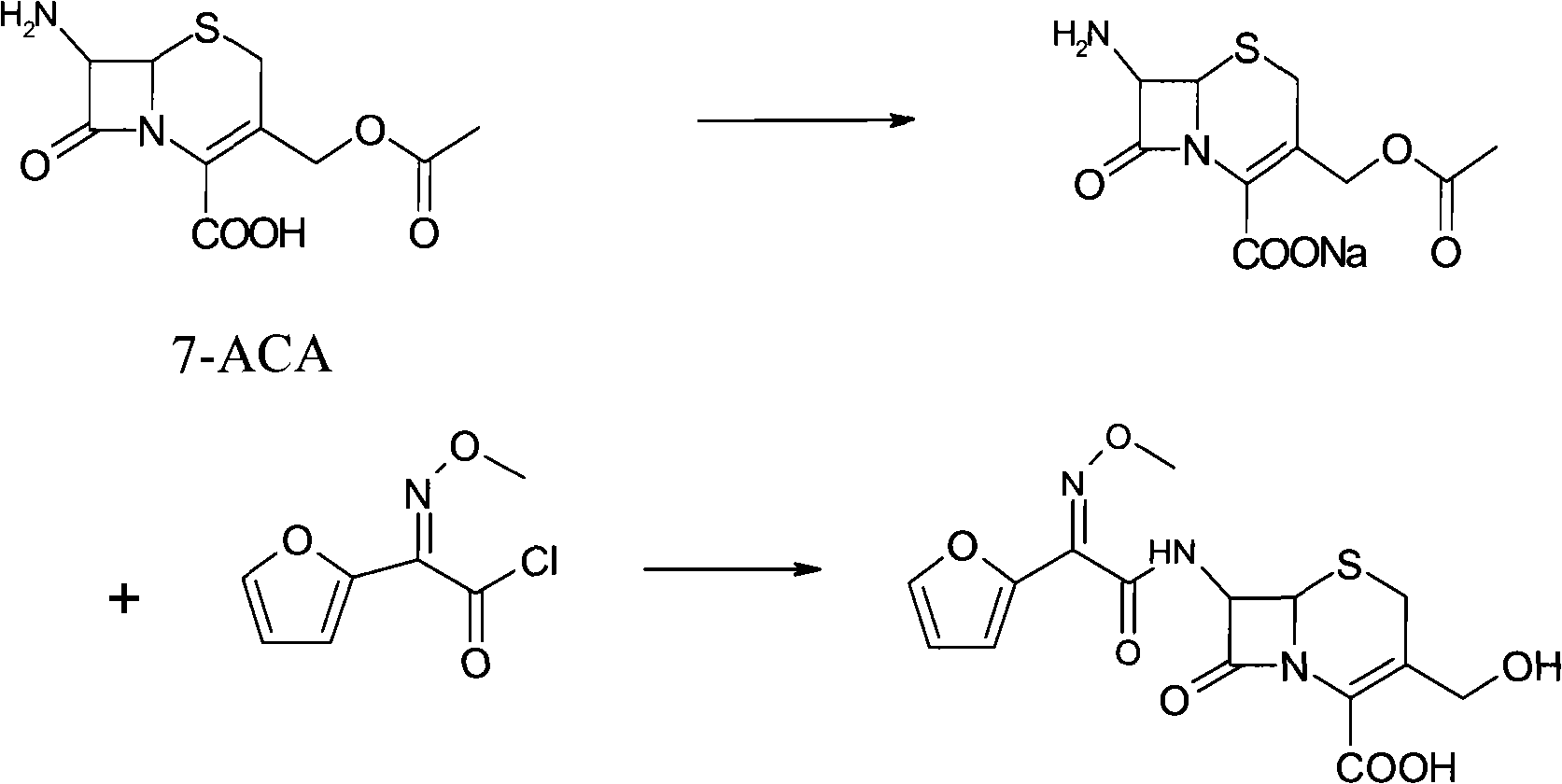
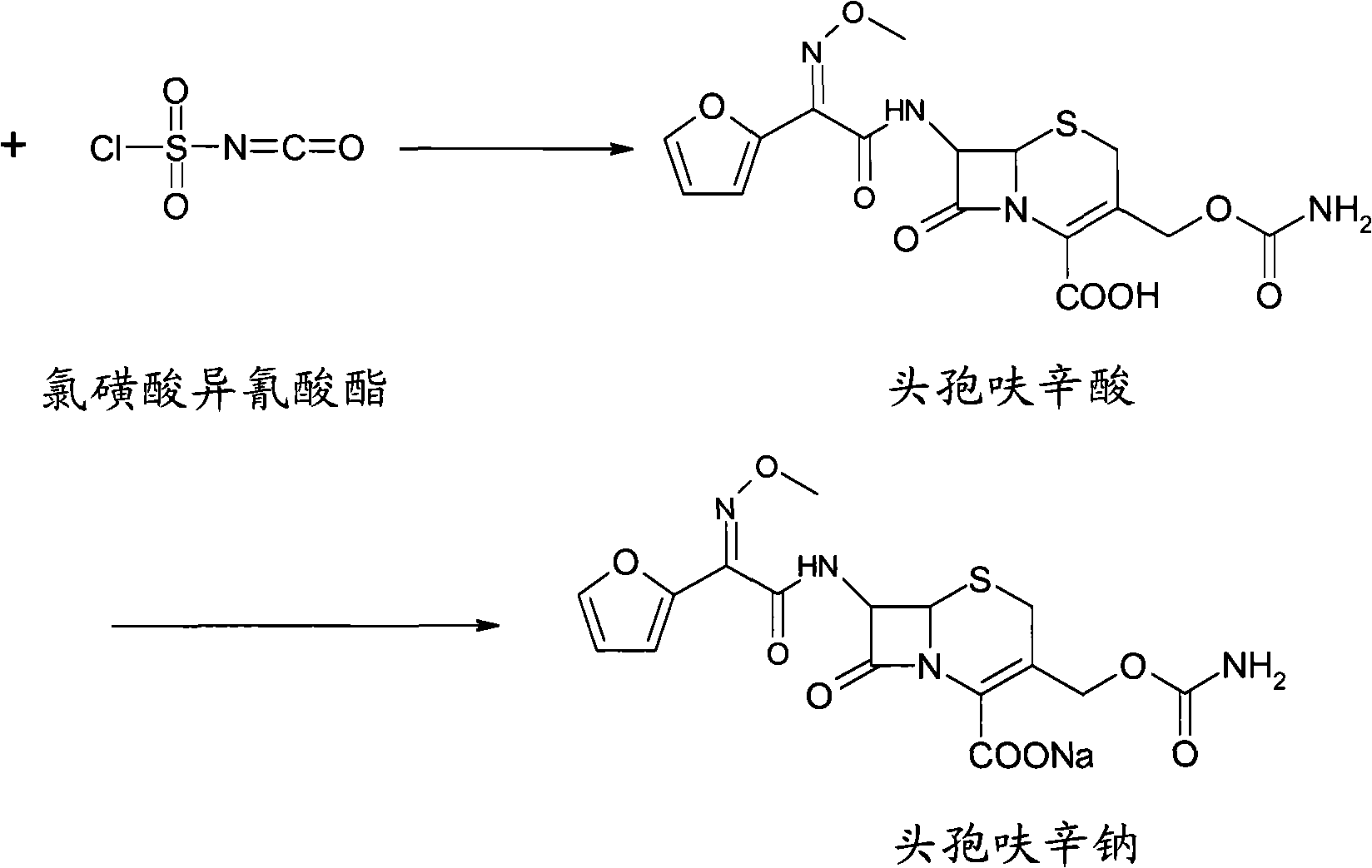
The invention provides cefuroxime sodium and a preparation method thereof. The preparation method comprises the following steps that: (1) 7-aminocephalosporanic acid reacts with 2-(furan-2-base)-2-(methoxyimino) acetyl chloride to produce 3-deacety-7-aminocephalosporanic acid; (2) crystallization is conducted after the 3-deacety-7-aminocephalosporanic acid reacts with chlorosulfonyl isocyanate to produce cefuroxime acid; and (3) the cefuroxime acid is salified to obtain the cefuroxime sodium, wherein solvent for crystallization is selected from one or more of petroleum ether, normal hexane, cyclohexane, solvent oil and tetrahydrofuran. Since the preparation method adopts solvents such as petroleum ether for crystallization in the process of the preparation of the cefuroxime acid, the invention has the advantages that the yield of the product is effectively improved, the product purity is further improved, the impurity content is reduced, the product quality is compliant with Chinese Pharmacopoeia of version 2005, the operation of the method is simple, the raw materials can be easily obtained, the cost is relatively low and the industrial production can be realized easily.
Description
technical field The invention belongs to the technical field of drug synthesis, and relates to a cephalosporin antibiotic and a preparation method thereof, in particular to cefuroxime sodium and a preparation method thereof. Background technique Cefuroxime sodium is the sodium salt of cefuroxime, which belongs to the second-generation cephalosporin antibiotics. It has a broad antibacterial spectrum and is effective against most Gram-negative bacteria, including Haemophilus influenzae, Neisseria gonorrhoeae, meningococcus, Escherichia coli, Klebsiella, Proteus mirabilis, Enterobacter, Citrobacter, Salmonella Bacteria, Shigella, and certain indole-positive denatured bacilli. The antibacterial spectrum against gram-positive bacteria is similar to that of cephalexin, mainly including staphylococci and streptococci. This product is relatively stable to β-lactamase, and its stability is no less than that of the third-generation cephalosporins. It is stable to almost all standard...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D501/34C07D501/04C07D501/12
Inventor 周自金符国庆
Owner LIVZON PHARM GRP INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com