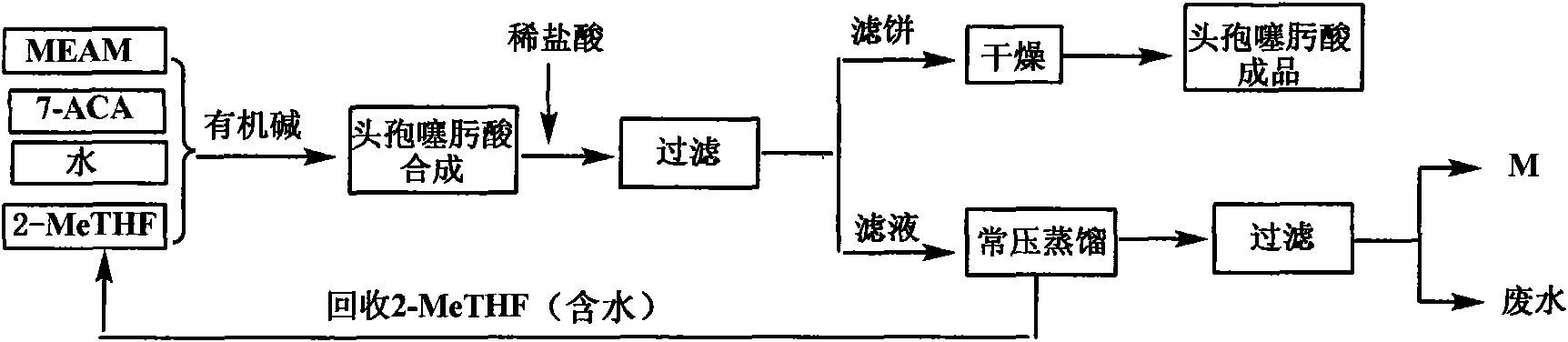
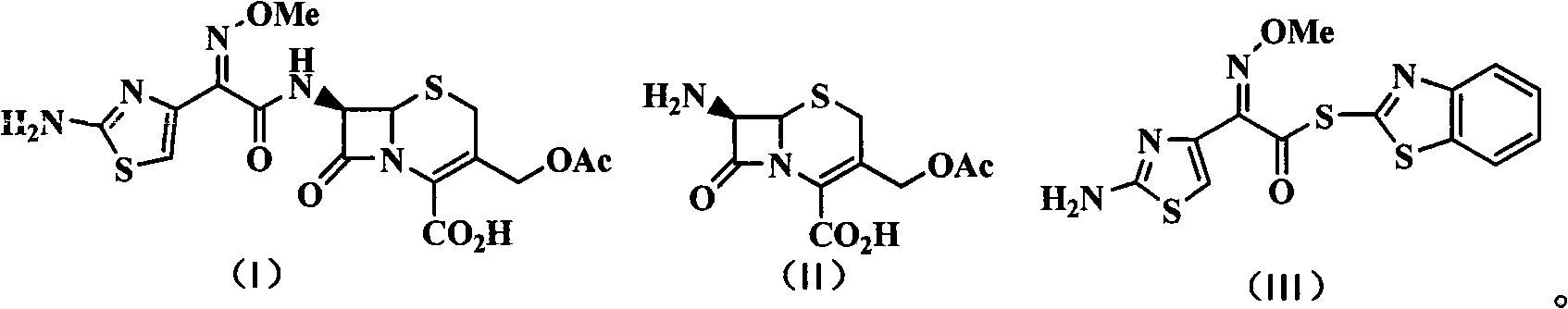
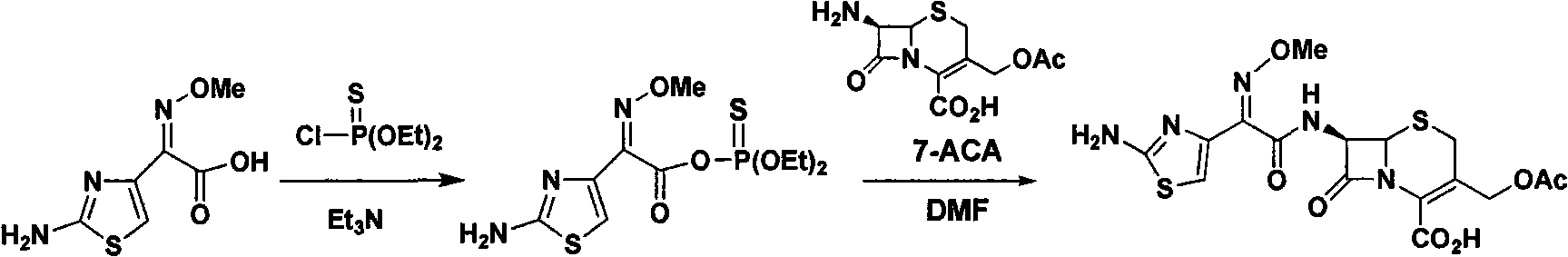
Green synthetic method for cefotaxime acid
A green synthesis technology of cefotaxime acid, applied in organic chemistry, antibacterial drugs, etc., can solve the problems of damage to the central nervous system and respiratory system, strong skin and mucous membrane irritation, low recovery rate of dichloromethane, etc., and achieve production The effect of low cost, good product quality and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The amount ratio of feeding material is 7-ACA: AE-active ester: organic base=1: 1.0: 0.4; Described organic base is triethylamine, and the consumption of 2-methyltetrahydrofuran is 4 times of 7-ACA feeding quality, The consumption of water is 0.6 times of 7-ACA feeding quality.
[0040] Control the temperature at 23±2°C (t), add 7-ACA (250.0g, 0.918mol) and AE-active ester (321.7g, 0.918mol) in a mixed solvent consisting of 2-MeTHF (1000g) and water (150g) , and triethylamine (37.1 g, 0.367 mol) was added simultaneously, and the reaction was stirred for 2 hours (T). The pH of the reaction solution was adjusted to 2.5±0.3 with 6 mol / L dilute hydrochloric acid, and a white solid was precipitated, filtered and dried to obtain 407.7 g of cefotaxime acid with a yield of 97.5% and a purity of 98.9% by HPLC. The filtrate was distilled under normal pressure to reclaim 1096g of the azeotropic liquid of 2-methyltetrahydrofuran and water (containing 2-MeTHF 980g), and the by-prod...
Embodiment 2
[0042]The molar ratio of the feed material is 7-ACA:AE-active ester:organic base=1:1.3:0.05; the organic base is pyridine. The specific addition is 250.0g (0.918mol) 7-ACA, 418.1gMEAM (1.193mol), 3.6g (0.046mol) pyridine, the consumption of 2-methyltetrahydrofuran is 1750g, and the consumption of water is 37.5g; It is 2mol / L, and the initial pH of crystallization is 2.85; t=20±2°C, T=2 hours.
[0043] Other operations are the same as in Example 1. 409.3 g of cefotaxime acid was obtained with a yield of 97.9% and a purity of 98.7% by HPLC. Recover 1770 g of azeotropic liquid of 2-methyltetrahydrofuran and water (containing 1743 g of 2-MeTHF), and 146.2 g of by-product 2-mercaptobenzothiazole.
Embodiment 3
[0045] The molar ratio of the feed material is 7-ACA:AE-active ester:organic base=1:1.8:0.5; the organic base is N-methylpyrrole. The specific addition amount is 250.0g (0.918mol) 7-ACA, 579.1g (1.652mol) MEAM, 37.7g (0.459mol) N-methylpyrrole, the amount of 2-methyltetrahydrofuran is 750g, and the amount of water is 250g; The concentration of the hydrochloric acid is 1mol / L, the initial pH of crystallization is 1.5; t=18±2°C, T=2.5 hours.
[0046] Other operations are the same as in Example 1. 411.0 g of cefotaxime acid was obtained with a yield of 98.3% and a purity of 98.5% by HPLC. Recover 818 g of azeotropic liquid of 2-methyltetrahydrofuran and water (containing 736 g of 2-MeTHF), and 145.1 g of by-product 2-mercaptobenzothiazole.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com