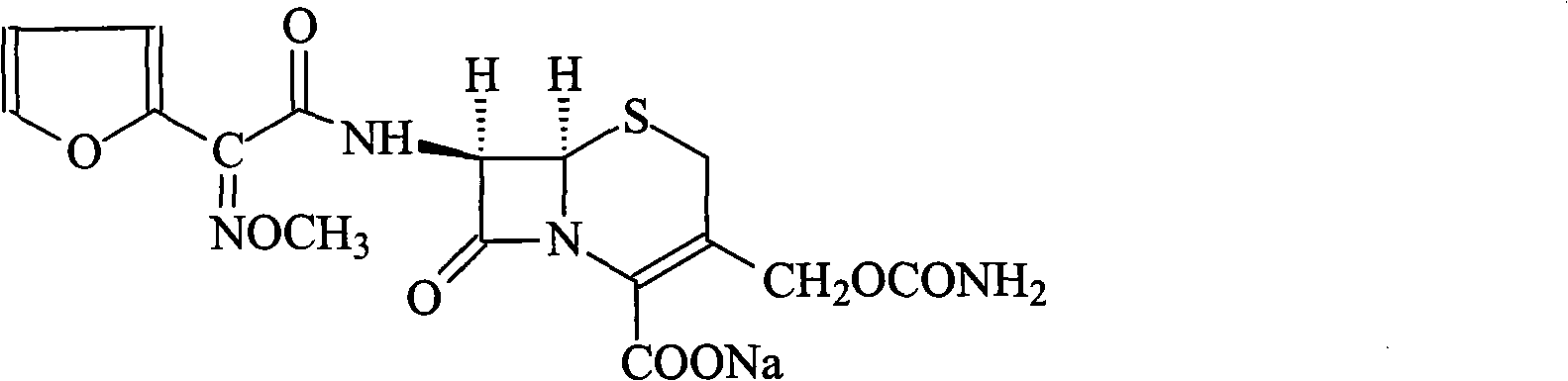
Method for synthesizing cefuroxime sodium
A cefuroxime sodium and synthetic method technology, applied in the field of drug synthesis, can solve problems such as high production cost, β-lactam ring damage, and reduced yield, and achieve stable product quality, simplified production steps, and reduced production costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Preparation of MDCC (decarbamoylcefuroxime acid):
[0035] In a 1000ml dry four-neck bottle, add 220ml of dichloromethane, PCI 5 35g, stir quickly for 1 hour, lower the temperature to -30°C, add 48ml of DMA (N.N-dimethylacetamide), 26g of SMIA, -10~-12°C, react for 80 minutes, wash with 3×110mil purified water, separate two Chloromethane phase is ready for use.
[0036] In another 250ml four-neck bottle, add 132ml of purified water, add 25.4g of 7-DACA, 0~2°C, dissolve until clear with 15% sodium hydroxide, add the above dichloromethane phase twice (previously Pour into the dissolved solution, keep the pH 6.5-7.0, react for 2 hours, separate the water phase, add 36ml of purified water to extract once, combine the water phase, when the temperature reaches 5-10°C, add 0.16g of disodium EDTA, 0.3g of sodium metabisulfite and 150ml of dichloromethane, then use 52ml of 16% hydrochloric acid to adjust the pH of the feed solution to 2.0, stir for 30 minutes and then filter, ...
Embodiment 2
[0042] Method is with embodiment 1,
[0043] Wherein water and dichloromethane mixed solvent are volume ratio 100ml: 200ml, adjust pH value to 1 with hydrochloric acid; Sodium bicarbonate lye is 1% sodium bicarbonate lye, absolute ethanol and acetone mixed solvent are volume ratio 2: 1.
Embodiment 3
[0045] Method is with embodiment 1,
[0046] Wherein water and dichloromethane mixed solvent are volume ratio 500ml: 100ml, adjust pH value to 2 with hydrochloric acid; Sodium bicarbonate lye is 10% sodium bicarbonate lye, absolute ethanol and acetone mixed solvent are volume ratio 1: 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com