Synthesis method of cefpodoxime proxetil intermediate
A technology of cefpodoxime axetil and a synthesis method is applied in the field of intermediate preparation of cefpodoxime axetil, can solve the problems of low yield, inability to proceed, low yield and purity, etc., and achieves high product yield and purity, high post-processing Uncomplicated and cost-effective
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
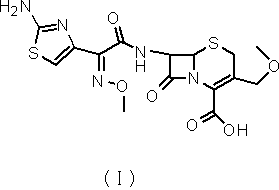
[0034] The intermediate of the present invention (6R,7R)-7-[2-(2-amino-4-thiazolyl)-(Z)-2-(methoxyimino)acetamido]-3-methoxymethyl- The synthetic method of 8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid specifically comprises the following steps:
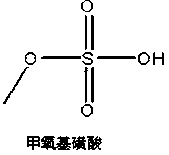
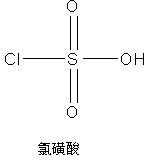
[0035] 1) Add 0.99 mol of methanol to the container, cool down to 10°C, add 0.8 mol of chlorosulfonic acid dropwise, and pass nitrogen gas for 4 hours after the drop to remove hydrogen chloride gas generated during the reaction, and concentrate to remove methanol to obtain methoxysulfonic acid.
[0036] 2) Add 50g of methoxysulfonic acid and 2g of dimethylformamide into the container, cool down to 15~20°C, add dropwise 11.4g of trimethyl borate / methanol mixed solution (mass ratio is 7:3), and cool down to 0~5℃; add 20g of 7-ACA, react for 2h; then add 10.8g of trimethyl borate / methanol mixed solution, react for 3h. The reaction solution was added dropwise to 200g of water and 30g of methanol, adjusted to pH=3.5 with co...
Embodiment 2
[0039] The identification of product in the embodiment of the present invention 1
[0040] Identification method: Bruker AvanceIII400MHZ superconducting NMR spectrometer;
[0041] Analytical method: LC (purity by liquid chromatography), Shimadzu LC-10ATVP. Agilent C-18 chromatographic column, mobile phase: methanol: buffer solution (potassium dihydrogen phosphate, disodium hydrogen phosphate, tetrabutylammonium bromide) = 1:3, flow rate 1mL / min, detection wavelength 240nm.
[0042] ESI-MS(m / z):
[0043] 428.0[M+1] + ,450.0[M+Na] + ,854.8[2M+1] + ,876.8[2M+Na] + ;
[0044] 1 HNMR: (400MHz, DMSO-d 6 )
[0045] δ3.20(s,3H),3.46(d,J=18,1H),3.58(d,J=18,1H),3.84(s,3H),
[0046] 4.17(s,2H),5.14(s,1H),5.75(s,1H),6.75(s,1H),9.62(d,J=18,1H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com