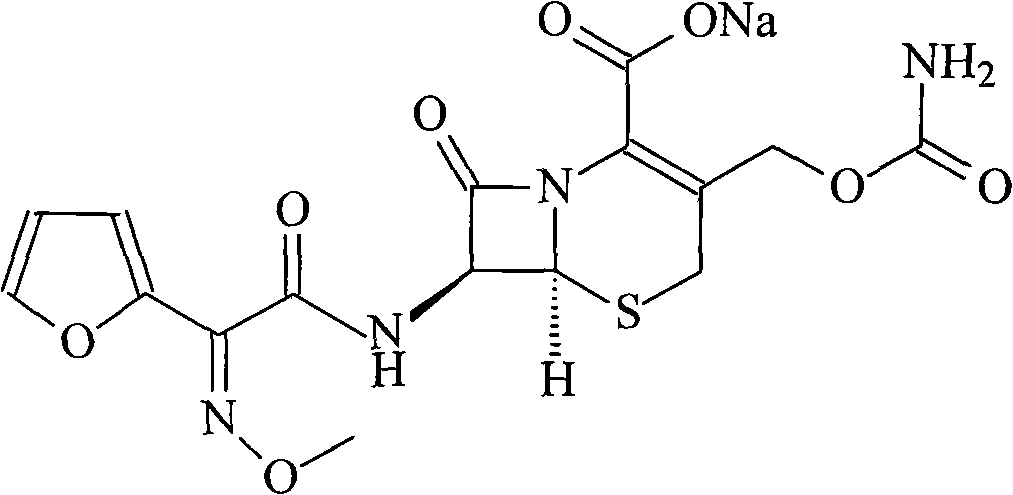
New method for preparing cefuroxime sodium compound
A technology of cefuroxime sodium and compounds, applied in the field of chemical synthesis, can solve the problems of low purity of finished products, difficulties in filtration and drying, and difficulty in separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
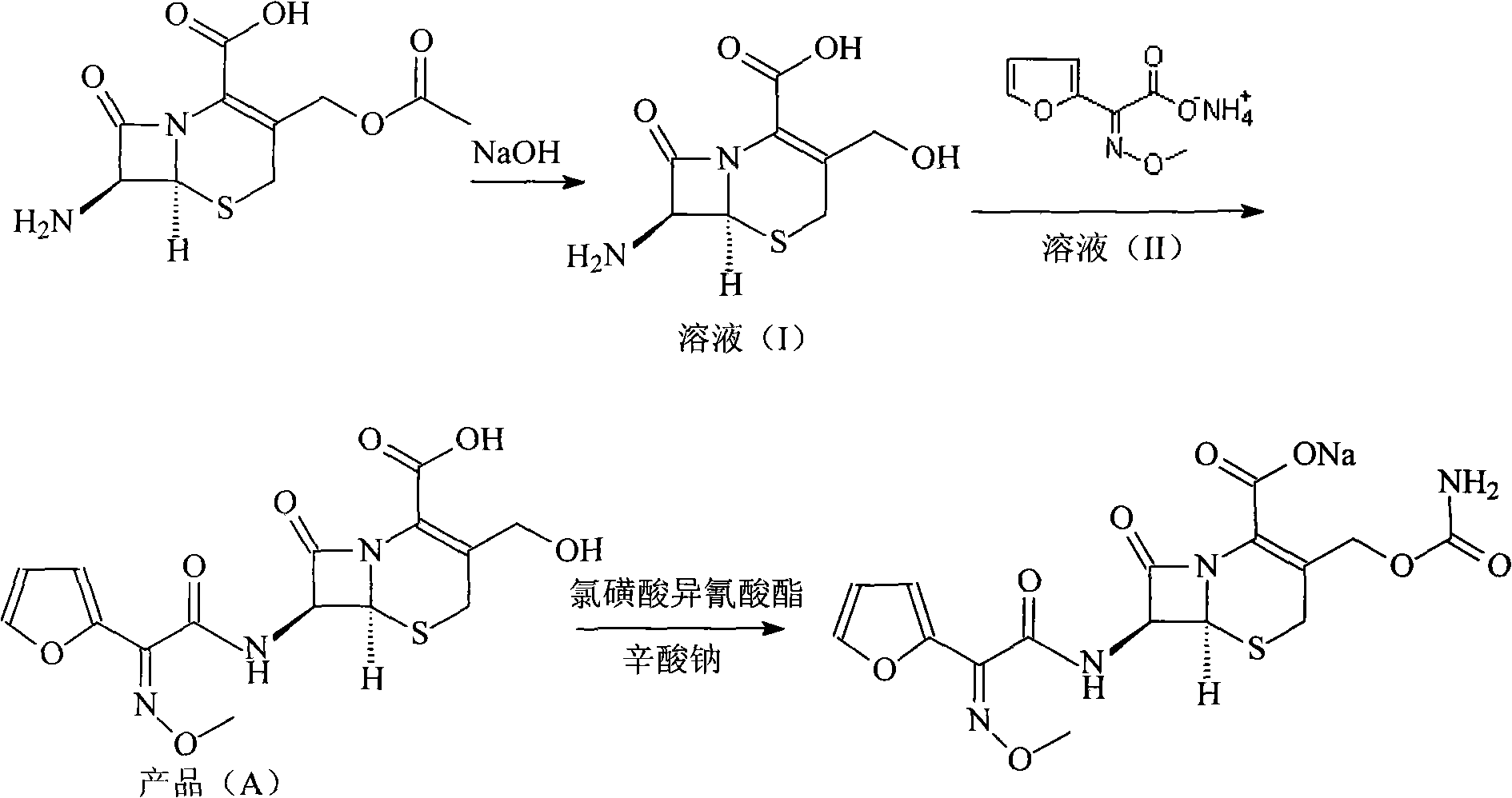
Image
Examples
Embodiment 1
[0031] 1. Deacetyl-7-aminocephalosporanic acid
[0032] Dissolve 272 grams of 7-aminocephalosporanic acid in a mixed solution of 1L methanol and 1L water, stir to lower the temperature, cool to -15°C, add 15% aqueous sodium hydroxide solution dropwise, and control the reaction temperature at -13°C to - 15°C, adjust the pH to 12.5, react for 2 hours, then adjust the pH to 9.5 with 2mol / L hydrochloric acid solution to obtain solution (1).
[0033] 2. 7-[2-(2-furyl)-2-(Z)-(methoxyimino)acetamido]-3-hydroxymethyl-ceph-3-ene-4-carboxylic acid
[0034] Dissolve 600 grams of triphosgene in 1L of toluene, add dropwise to 186 grams of (Z)-methoxyiminofuran acetate ammonium salt and 556 grams of triphenylphosphine in 2L of toluene at 5°C, and keep warm at 5°C React for 3 hours to obtain solution (2); add solution (2) dropwise to solution (1) for mixed reaction, adjust pH=9.5 with 15% sodium hydroxide solution during the reaction, control the reaction temperature at 5°C, add dropwise A...
Embodiment 2
[0038] 1. Deacetyl-7-aminocephalosporanic acid
[0039] Dissolve 136 grams of 7-aminocephalosporanic acid in a mixed solution of 0.5L methanol and 0.5L water, stir to lower the temperature, cool to -13°C, add dropwise 5% aqueous sodium hydroxide solution, and control the reaction temperature at -13°C to -15°C, adjust the pH to 11.5, react for 1 hour, and then adjust the pH to 9.5 with 2 mol / L hydrochloric acid solution to obtain solution (1).
[0040] 2. 7-[2-(2-furyl)-2-(Z)-(methoxyimino)acetamido]-3-hydroxymethyl-ceph-3-ene-4-carboxylic acid
[0041] Dissolve 300 grams of triphosgene in 0.5 L of toluene, add dropwise to 93 grams of (Z)-methoxyiminofuran acetate ammonium salt and 278 grams of triphenylphosphine in 1 L of toluene at 8 ° C, and keep the temperature for 8 ℃ for 2 hours, to obtain solution (2); solution (2) was added dropwise to solution (1) for mixed reaction, and 5% sodium hydroxide solution was used to adjust pH=10 in the reaction, and the reaction temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com