Cefuroxime sodium compound entity and application thereof
A technology of cefuroxime sodium and compound, which is applied in the field of medicine and achieves the effects of good stable storage, good sliding property, and convenient storage and transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
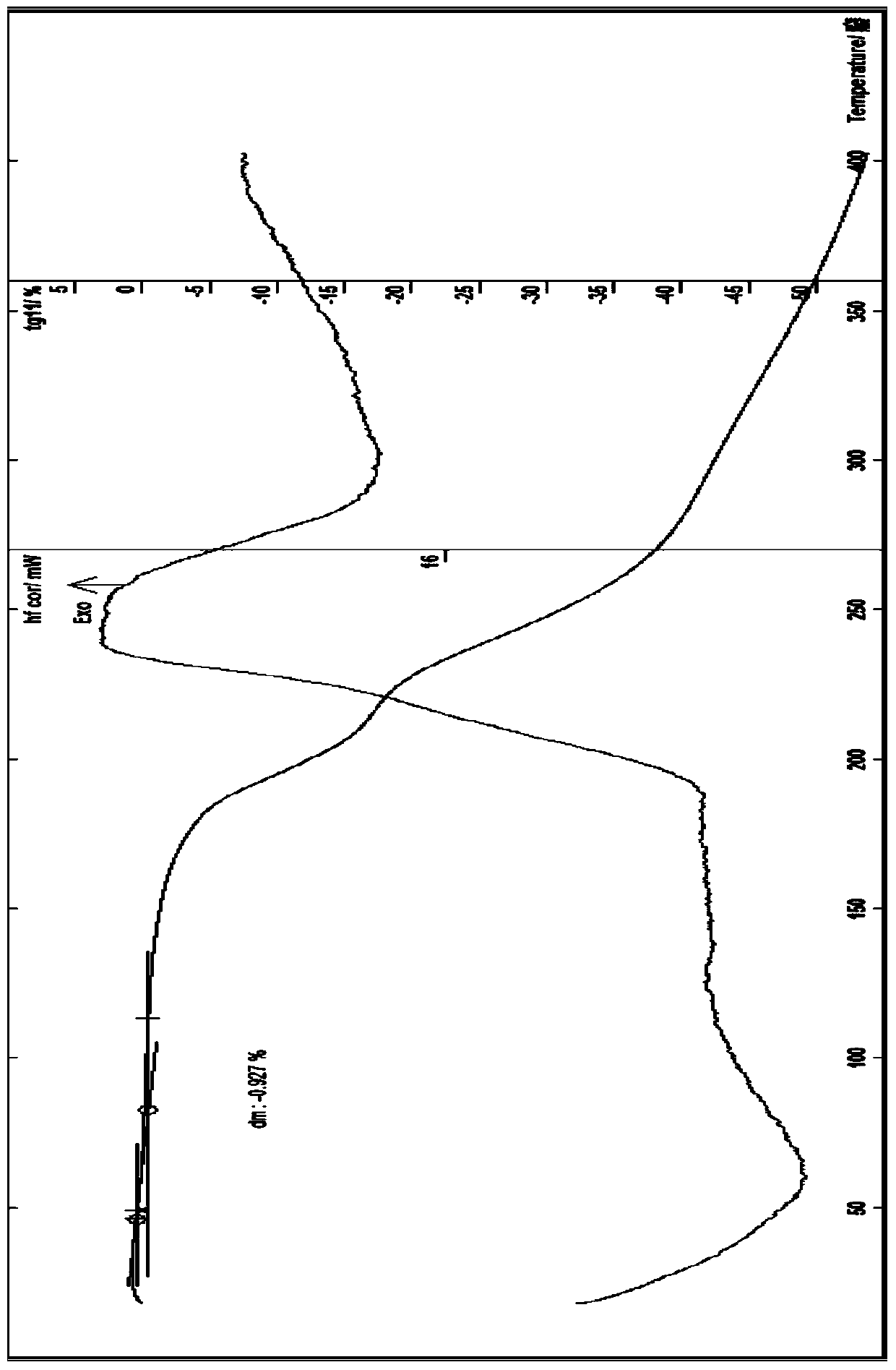
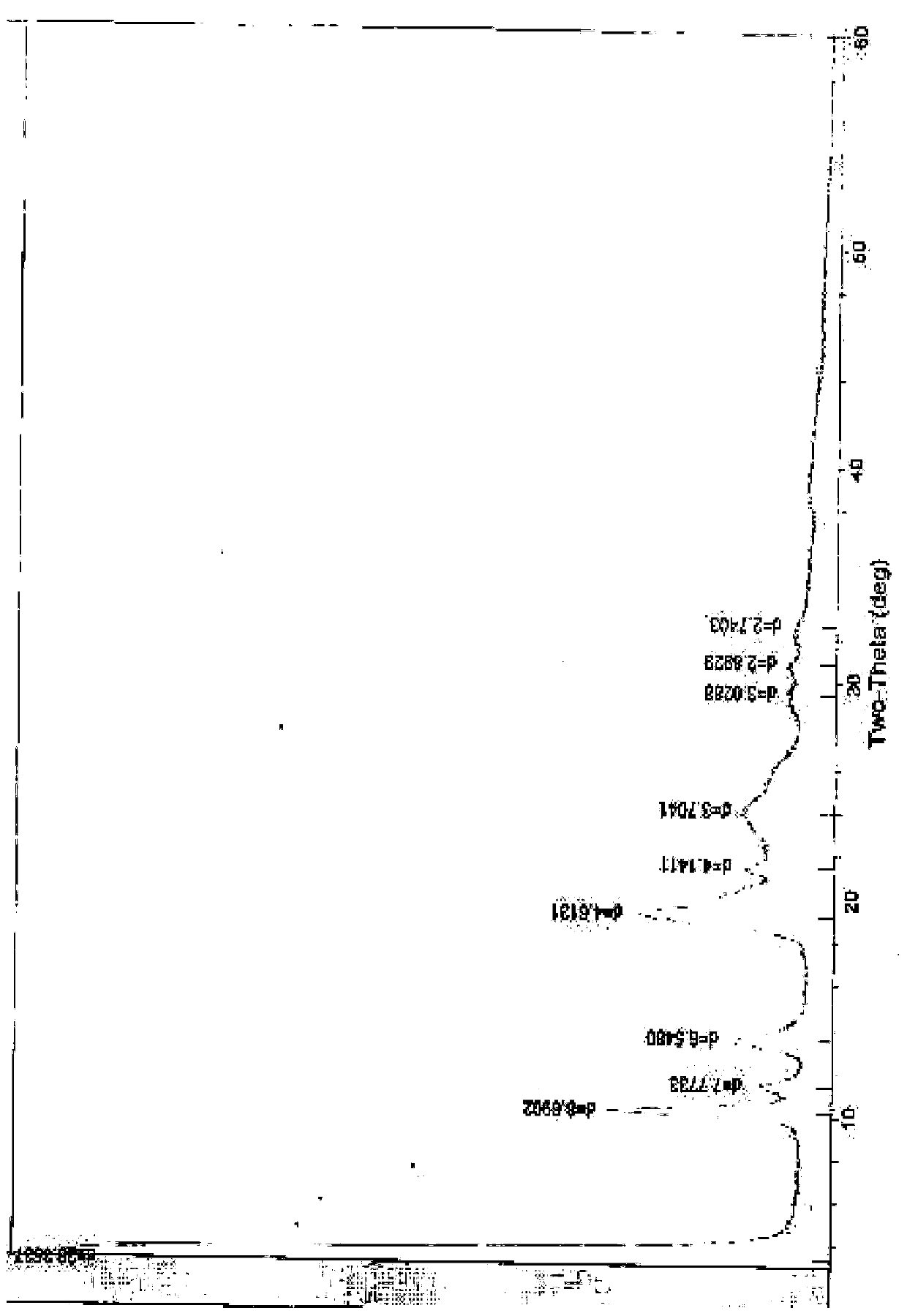
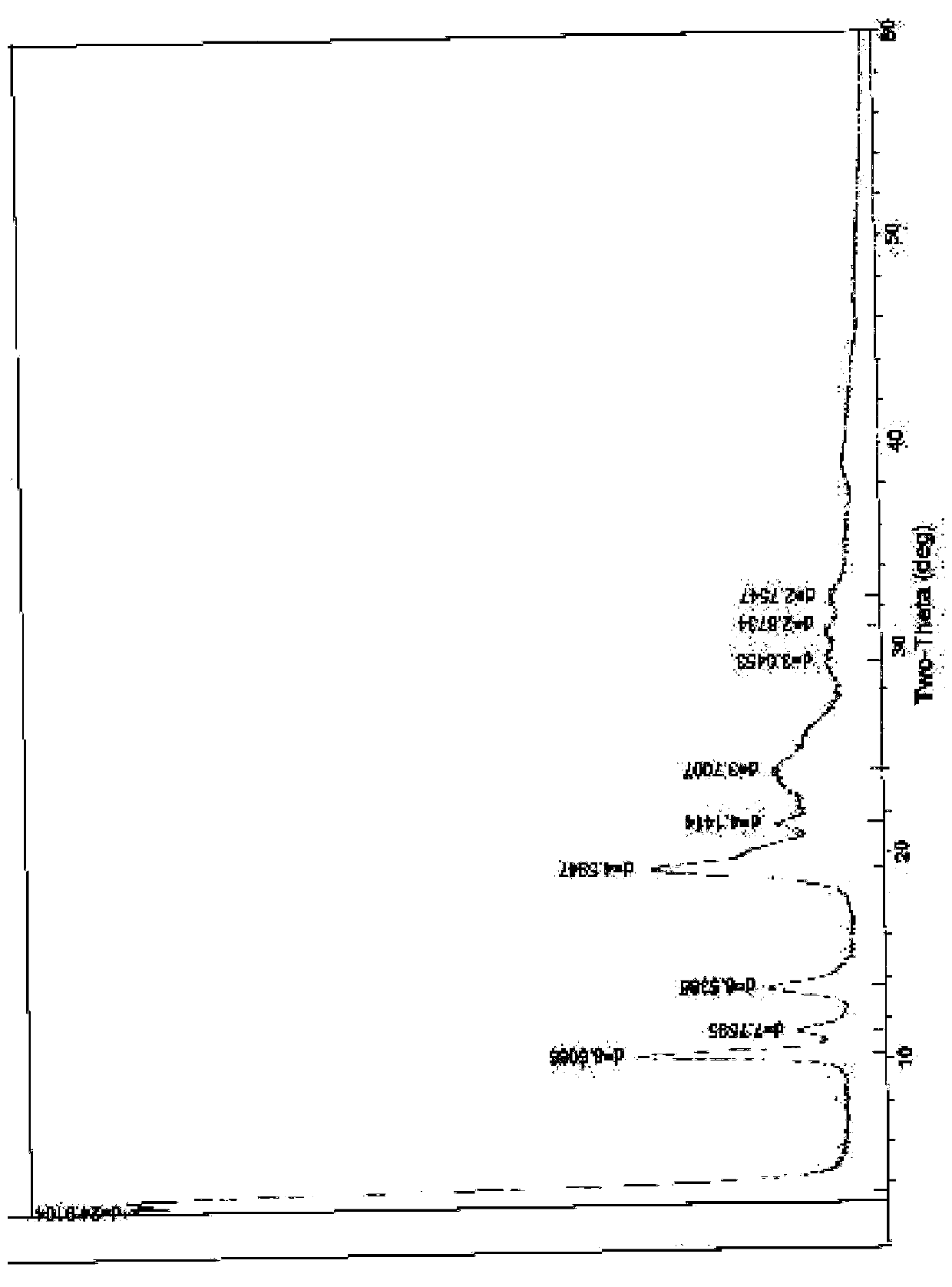
[0063] The preparation of embodiment 1 cefuroxime sodium 0.25 hydrate
[0064] At room temperature, add 10.6g of high-purity cefuroxime acid, 180ml of acetone, 10ml of ethanol, and 5ml of water into a 500ml flask, stir to dissolve, add 0.06g of activated carbon, stir for 30 minutes, filter with suction, and dissolve in the filtrate at 4°C Add 60ml of a mixed solution of sodium isooctanoate and ethanol (containing 4.72g of sodium isooctanoate) dropwise, stir, and place below 4°C to allow the solid to fully separate out, filter with suction, wash twice with a small amount of chloroform and twice with a small amount of ethanol, and filter with suction to obtain Dissolve the solid with a small amount of water, then use 260ml of ethanol, 20ml of isopropanol, and 20ml of acetone as solvents for recrystallization, place it below 4°C to allow the crystals to fully separate out, filter with suction, rinse with a small amount of ethanol and chloroform alternately, and filter with suction...
Embodiment 2
[0065] The preparation of embodiment 2 cefuroxime sodium 0.25 hydrate
[0066]At room temperature, add 20g of cefuroxime acid, 180ml of acetone, 10ml of methanol, and 5ml of water into a 1000ml flask, stir to dissolve, add 0.2g of activated carbon, stir for 30 minutes, filter with suction, add dropwise a mixed solution of sodium isooctanoate and ethanol to the filtrate (Containing 9.42g of sodium isooctanoate) 150ml, place it below 4°C, let the solid fully separate out, filter with suction, wash with a small amount of ethanol for 3 times, filter with suction, dissolve the obtained solid with a small amount of water, add 300ml of ethanol, 20ml of isopropanol, 20ml of isopropanol Recrystallize 5ml of ether, place it below 5°C to allow the crystals to fully separate out, filter with suction, wash twice with a small amount of dichloromethane, wash twice with a small amount of ethanol, filter with suction, dilute the obtained solid at about 20°C and blow dry for 1 day, then Vacuum ...
Embodiment 3
[0067] The preparation of embodiment 3 cefuroxime sodium 0.25 hydrate
[0068] Add 10g of cefuroxime acid and 20ml of water to a 500ml flask, add an appropriate amount of 95% ethanol and stir until dissolved, add a saturated aqueous solution of sodium bicarbonate dropwise at 10°C until the pH is about 8.9, stir to dissolve, add 0.1g of activated carbon, Stir for 30 minutes, filter with suction, slowly add 10ml of chloroform and 300ml of ethanol dropwise to the filtrate, place it below 10°C, let the solid fully separate out, filter with suction, wash with a small amount of chloroform and ethanol twice, filter with suction, dissolve the obtained solid with a small amount of water, Then use 220ml of ethanol, 10ml of isopropanol, 2ml of chloroform, and 2ml of ethyl acetate as crystallization solvents for recrystallization, place below 8°C to allow the crystals to fully separate out, filter with suction, and wash with a small amount of dichloromethane, ethyl acetate, and ethanol in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com