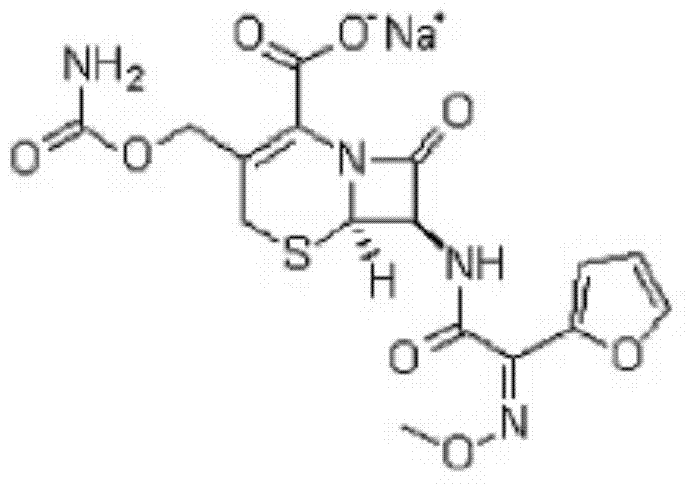
Cefuroxime sodium powder preparation for injection
A technology of sodium fluorescein powder and cefuroxime acid, applied in the field of medicine, can solve the problems of residual solvent, instability, inclusion and the like, and achieve the effects of less residual solvent, high stability and less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
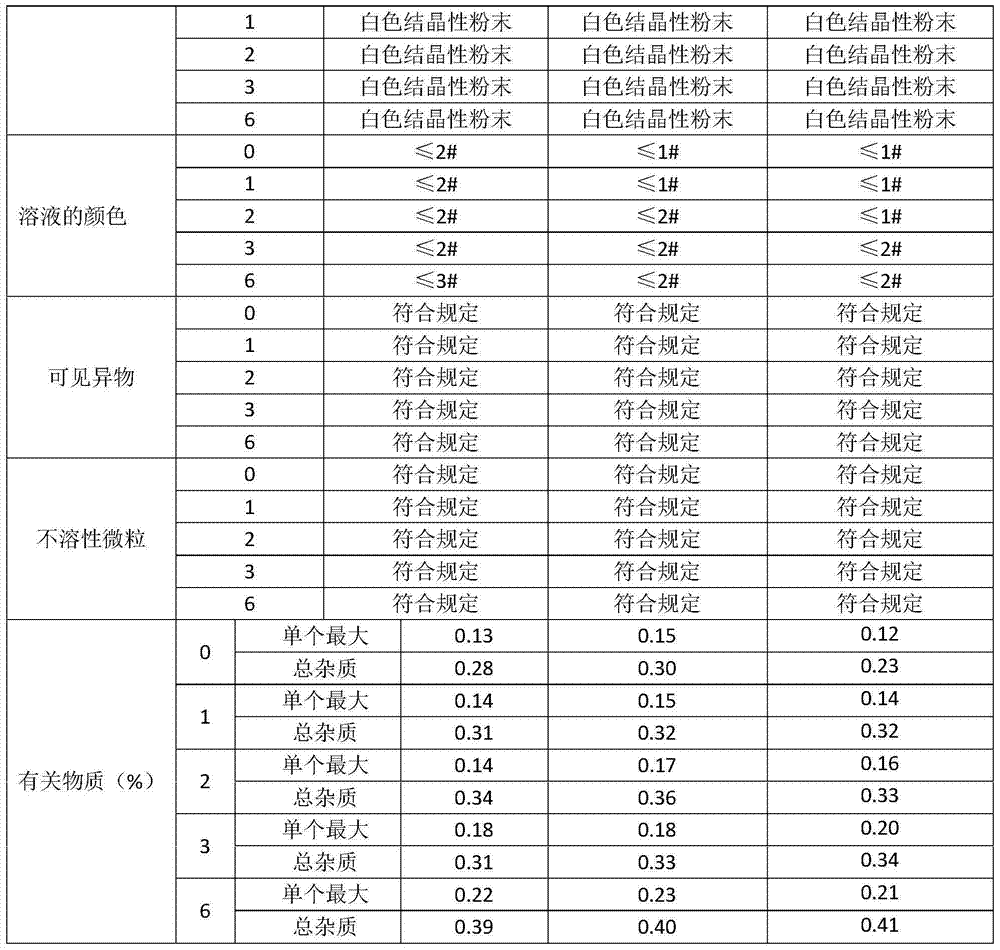
Examples
Embodiment 1
[0031] (1) add weighed methanol 153kg, anhydrous sodium acetate 18kg in the stainless steel reaction tank, stir to make solid material all dissolve, set aside;
[0032] (2) Add 576 kg of acetone and 60 kg of water into a cleaned and dried stainless steel reaction tank, add 85 kg of cefuroxime acid under stirring, and stir until dissolved under nitrogen protection. Add 6 kg of activated carbon into the dissolving tank, and stir and decolorize at 10° C. for 30 minutes. Filter the dissolved and decolorized solution into a sterile crystallization tank; add the prepared acetone and water mixed washing solution into the dissolution tank, and filter the washing solution into the crystallization tank;
[0033] (3) Control the feed liquid in the crystallization tank at 10-15°C, control the stirring speed at 100 rpm, control the nitrogen pressure <0.2MPa, add sodium acetate solution dropwise, and finish adding in 60-80 minutes; flow acceleration rate according to the table Add solvent ...
Embodiment 2
[0038] (1) add weighed ethanol 172kg, anhydrous sodium acetate 22kg in the stainless steel reaction tank, stir to make solid material all dissolve, set aside;
[0039] (2) Add 597kg of ethanol and 60kg of water into a cleaned and dried stainless steel reaction tank, add 78kg of cefuroxime acid under stirring, and stir until dissolved under nitrogen protection. Add 6 kg of activated carbon into the dissolving tank, and stir and decolorize at 10° C. for 30 minutes. Filter the dissolved and decolorized solution into a sterile crystallization tank; add the prepared acetone and water mixed washing solution into the dissolution tank, and filter the washing solution into the crystallization tank;
[0040] (3) Control the feed liquid in the crystallization tank at 10-15°C, control the stirring speed at 100 rpm, control the nitrogen pressure <0.2MPa, add sodium acetate solution dropwise, and finish adding in 60-80 minutes; flow acceleration rate according to the table Add solvent etha...
Embodiment 3
[0045] (1) add weighed ethanol 163kg, anhydrous sodium acetate 20kg in the stainless steel reaction tank, stir to make solid material all dissolve, set aside;
[0046] (2) Add 552 kg of ethanol and 60 kg of water into a cleaned and dried stainless steel reaction tank, add 92 kg of cefuroxime acid under stirring, and stir until dissolved under nitrogen protection. Add 6 kg of activated carbon into the dissolving tank, and stir and decolorize at 10° C. for 30 minutes. Filter the dissolved and decolorized solution into a sterile crystallization tank; add the prepared acetone and water mixed washing solution into the dissolution tank, and filter the washing solution into the crystallization tank;
[0047] (3) Control the feed liquid in the crystallization tank at 10-15°C, control the stirring speed at 100 rpm, control the nitrogen pressure <0.2MPa, add sodium acetate solution dropwise, and finish adding in 60-80 minutes; flow acceleration rate according to the table Add solvent e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com