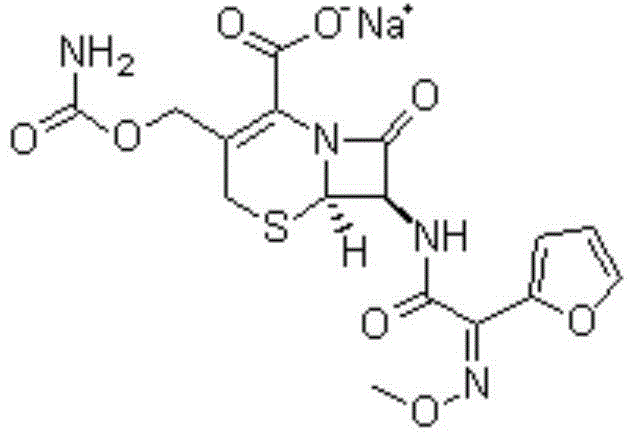
Novel cefuroxime sodium compound
A technology of cefuroxime sodium and its compound, which is applied in the field of medicine, and can solve problems such as poor solubility, slow dissolution rate of cefuroxime sodium, and incomplete dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 prepares new cefuroxime sodium compound of the present invention
[0033] Add 1000g of crude cefuroxime sodium into a 15L three-necked flask, add 7000mL of water and 1200mL of cycloethanol, stir and heat to reflux to dissolve, then add 600mL of ethyl acetate to the solution, then slowly cool down to 10°C, stir and crystallize, filter , washed with ethyl acetate, and vacuum-dried at 50° C. to obtain 956 g of dry cefuroxime sodium, with a yield of 95.6%.
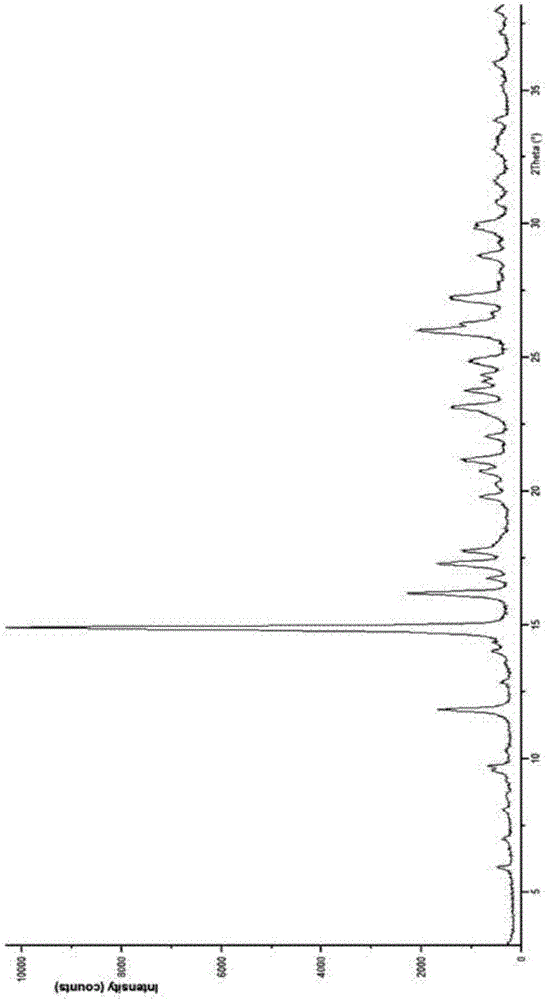
[0034] The X-ray powder diffraction pattern of the new cefuroxime sodium compound at the reflection angle 2θ is (with an accuracy of ±0.1°): 11.78°, 14.86°, 16.12°, 17.24°, 19.74°, 21.11°, 23.07°, 23.72° , 24.08°, 24.33°, 24.82°, 25.96°, 26.26°, 27.16°, 28.77°, 29.82° have characteristic absorption peaks, such as figure 1 shown.
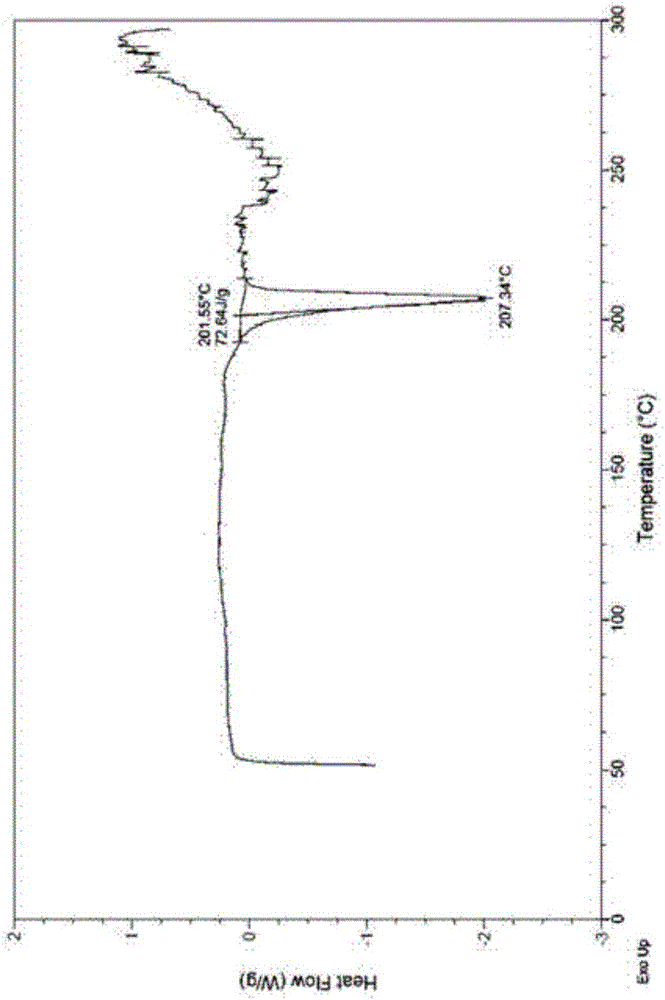
[0035] Its DSC spectrum has an endothermic peak near 207.34°C (with an accuracy of ±2°C), such as figure 2 shown.
Embodiment 2
[0036] Embodiment 2 prepares the new cefuroxime sodium compound of the present invention
[0037] Add 1,000 g of crude cefuroxime sodium into a 15 L three-necked flask, add 6,000 mL of water and 800 mL of cyclohexanol, stir and heat to reflux to dissolve, then add 400 mL of ethyl acetate to the solution, then slowly cool down to 15°C, stir and crystallize, Filter, wash with ethyl acetate, and dry under vacuum at 40°C to obtain 917 g of dry cefuroxime sodium, with a yield of 91.7%.
Embodiment 3
[0038] Embodiment 3 prepares new cefuroxime sodium compound of the present invention
[0039]Add 1,000 g of crude cefuroxime sodium into a 15 L three-necked flask, add 8,000 mL of water and 1,600 mL of cyclohexanol, stir and heat to reflux for dissolution, then add 800 mL of ethyl acetate to the solution, then slowly cool down to 0°C, stir and crystallize, Filter, wash with ethyl acetate, and dry under vacuum at 60°C to obtain 922 g of dry cefuroxime sodium, with a yield of 92.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com