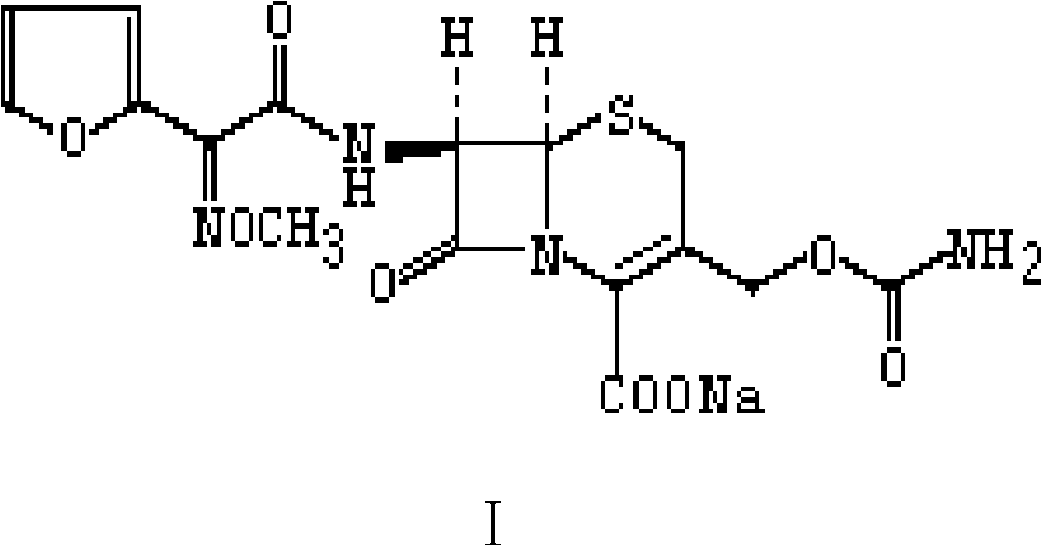
Preparation method of stable cefuroxime sodium
A technology of cefuroxime sodium and cefuroxime sodium, applied in the field of medicine, can solve the problems of not being able to store conditions in a cool and cool warehouse, slowing product discoloration, poor stability, etc., and achieving high yield, low product impurities, and stability. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
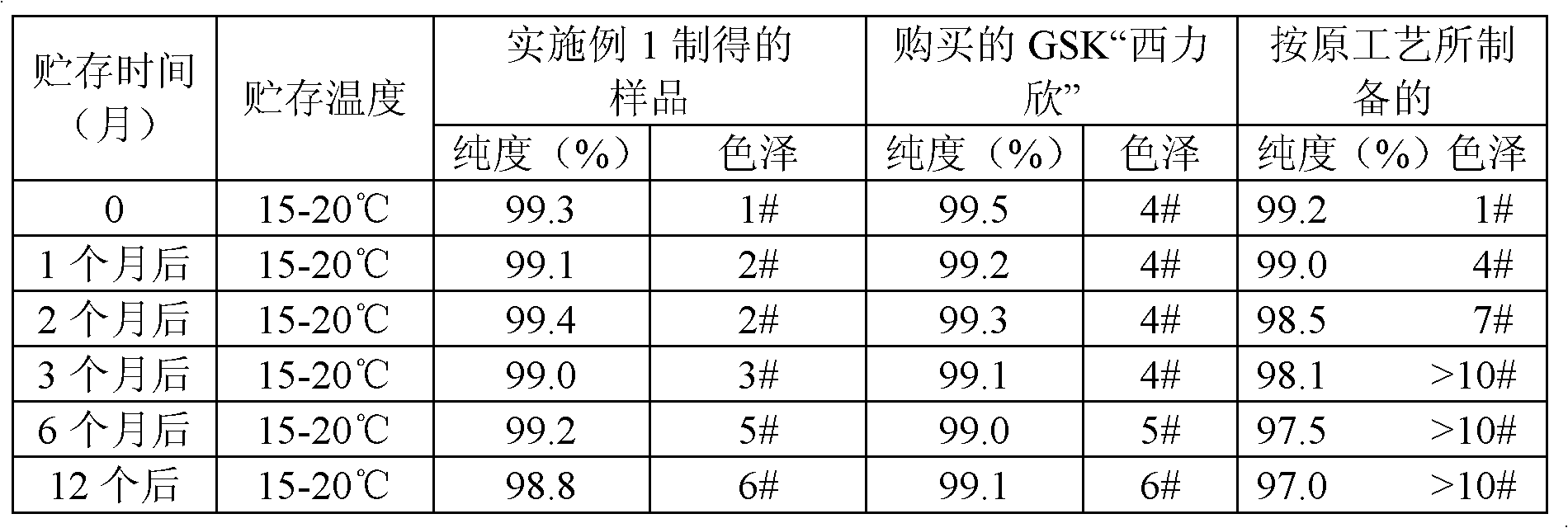
[0017] In a jacketed reaction flask, add 100 g of cefuroxime acid, 300 ml of acetone, and 50 ml of purified water, stir until completely dissolved, add activated carbon, stir and decolorize at room temperature for 30 minutes, and filter; a mixture consisting of acetone and a small amount of purified water The mixture was washed, the filtrate was combined, absolute ethanol was added, the temperature was controlled at 25°C, and sodium lactate (74 g) and sodium acetate (11 g) (dissolved in 100 ml of absolute ethanol) were added dropwise. After the dropwise addition, the reaction was stirred for 30 min. Then, add 800 ml of acetone dropwise within 15min, cool to 10°C and stir to grow crystals, filter, stir and wash with a mixture of acetone+purified water+ethanol; wash with appropriate amount of acetone, measure the pH value of cefuroxime sodium More than 6 is sufficient, and vacuum-drying below 45° C. until the moisture content is qualified to obtain 97 g of cefuroxime sodium with ...
Embodiment 2
[0021] In a jacketed reaction flask, add 100 grams of cefuroxime acid, 500 ml of acetone, and 70 ml of purified water, stir until completely dissolved, add activated carbon, stir and decolorize at room temperature for 30 minutes, and filter; The mixed solution was washed, the filtrate was combined, absolute ethanol was added, the temperature was controlled at 35°C, and sodium lactate (86 g) and sodium acetate (9 g) (dissolved in 100 ml of absolute ethanol) were added dropwise. After the dropwise addition, the reaction was stirred for 30 min. Subsequently, 1000 ml of acetone was added dropwise in 45min, cooled to 10°C, stirred and grown for crystal growth, filtered, and washed with a mixture of acetone+purified water+ethanol; 5. Vacuum drying below 45° C. until the moisture content is qualified to obtain 98 g of cefuroxime sodium with a purity of 99.5%.
Embodiment 3
[0023] In a jacketed reaction flask, add 100 grams of cefuroxime acid, 400 ml of acetone, and 40 ml of purified water, stir until completely dissolved, add activated carbon, stir and decolorize at room temperature for 30 minutes, and filter; The mixed solution was washed, the filtrate was combined, absolute ethanol was added, the temperature was controlled at 15°C, and sodium lactate (70 g) and sodium acetate (22 g) (dissolved in 100 ml of absolute ethanol) were added dropwise. After the dropwise addition, the reaction was stirred for 30 min. Then, add 1200 ml of acetone dropwise within 15min, cool to 10°C and stir to grow crystals, filter, stir and wash with a mixture of acetone+purified water+ethanol; wash with appropriate amount of acetone, measure the pH value of cefuroxime sodium 6.9, vacuum drying below 45°C until the moisture content is qualified to obtain 97 g of cefuroxime sodium with a purity of 99.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com