Pharmaceutical packaging composition for injection and preparation method thereof
A technology for injection and composition, which is applied in the field of medicine, can solve the problems of rubber stopper compatibility, affect the quality of medicines, and complex formulations, etc., achieve strong resistance to water vapor permeability, ensure production efficiency, elasticity and flexibility good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
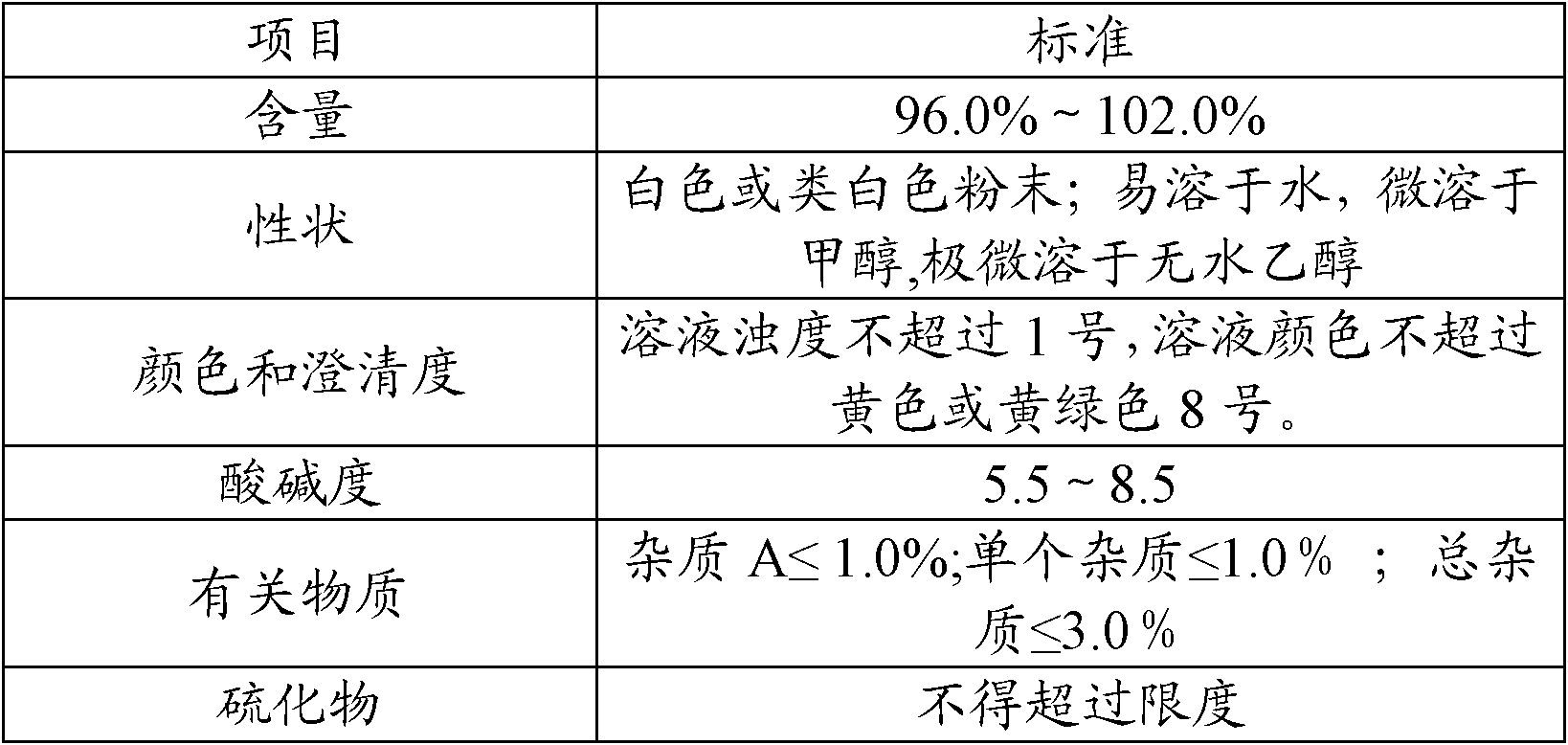
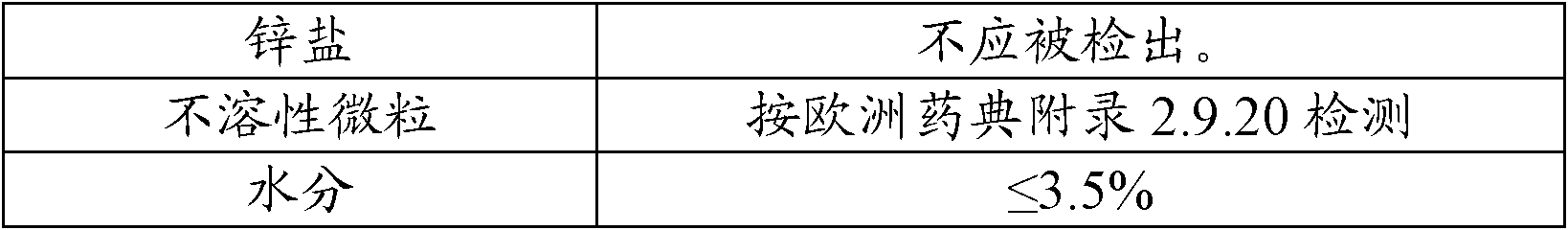
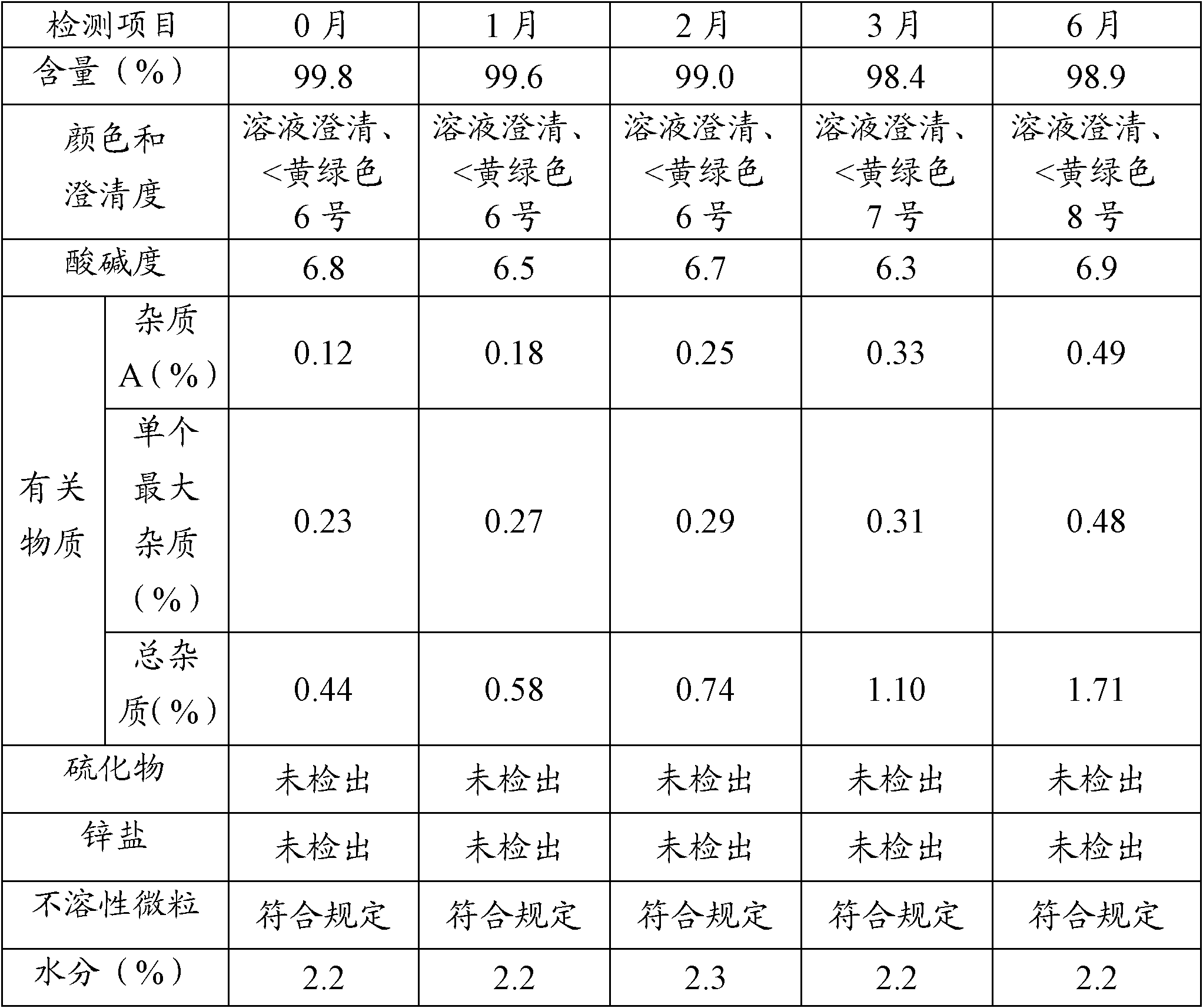
[0034] A packaging composition of cefuroxime sodium for injection: an aluminum-plastic combination cover containing a rubber stopper, a sterile antibiotic glass bottle and a sterile powder of cefuroxime sodium for injection, and the weight of the sterile powder of cefuroxime sodium for injection is In terms of cefuroxime 0.75g, its preparation method comprises the following steps:
[0035] (1) To prepare film-coated rubber stoppers, first evaporate p-xylene at 150°C, then crack it at 680°C to generate stable active monomers, and finally the active monomers enter the deposition chamber at room temperature for polymerization and deposition. It is adsorbed on the rubber plug and polymerized to form a film, and the remaining gas is captured by a cold trap to prevent the deposit from entering the vacuum pump to obtain a film-coated rubber plug;
[0036] (2) Packing: put the sterile powder of cefuroxime sodium for injection into a sterile antibiotic glass bottle in the aseptic works...
Embodiment 2
[0039] A kind of packaging composition of cefoxitin sodium for injection: the aluminum-plastic combination cover that contains coated rubber stopper, sterile antibiotic glass bottle and cefoxitin sodium for injection aseptic powder, the weight of cefoxitin sodium for injection aseptic powder is In terms of cefoxitin 1.0g, its preparation method may further comprise the steps:
[0040] (1) To prepare film-coated rubber stoppers, first evaporate p-xylene at 150°C, then crack it at 680°C to generate stable active monomers, and finally the active monomers enter the deposition chamber at room temperature for polymerization and deposition. It is adsorbed on the rubber plug and polymerized to form a film, and the remaining gas is captured by a cold trap to prevent the deposit from entering the vacuum pump to obtain a film-coated rubber plug;
[0041] (2) Packing: in the aseptic workshop, put the sterile powder of cefoxitin sodium for injection into a sterile antibiotic glass bottle, ...
Embodiment 3
[0044] A kind of packaging composition of ceftriaxone sodium for injection: the aluminum-plastic combination cover that contains coated rubber stopper, sterile antibiotic glass bottle and ceftriaxone sodium for injection aseptic powder, the weight of ceftriaxone sodium for injection aseptic powder is In terms of ceftriaxone 1.0g, its preparation method may further comprise the steps:
[0045] (1) To prepare film-coated rubber stoppers, first evaporate p-xylene at 150°C, then crack it at 680°C to generate stable active monomers, and finally the active monomers enter the deposition chamber at room temperature for polymerization and deposition. It is adsorbed on the rubber plug and polymerized to form a film, and the remaining gas is captured by a cold trap to prevent the deposit from entering the vacuum pump to obtain a film-coated rubber plug;
[0046] (2) Packing: in the aseptic workshop, put the sterile powder of ceftriaxone sodium for injection into a sterile antibiotic glas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com