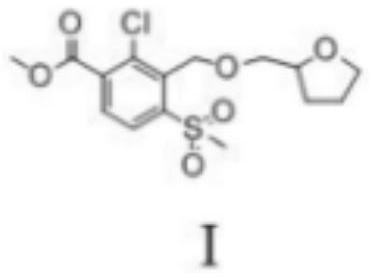
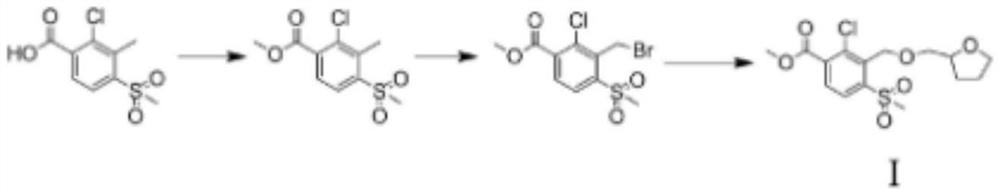
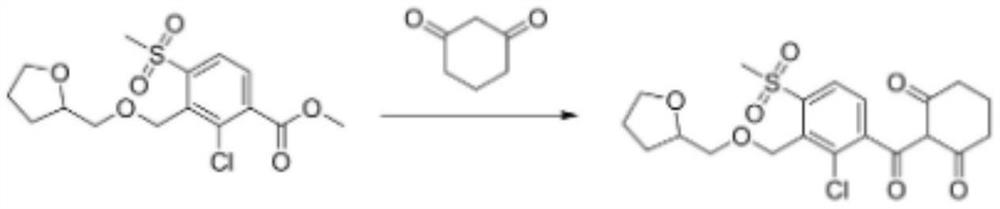
A kind of preparation method of furansulcotrione
A technology of furansulcotrione and tetrahydrofuran, which is applied in the field of compound synthesis, can solve the problems of lowering furansulcotrione yield, complex reaction steps, complicated preparation process, etc., and achieves environmental protection, less reaction process, and simple preparation process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Take 20ml of methanol into a three-neck bottle, then weigh 3g of 2-chloro-3-methyl-4-methanesulfonylbenzoic acid and add it, then put it into the rotor, add 10ml of methanol, install a reflux device and a thermometer, and The mouth is coated with vacuum ester, so that the whole reaction is carried out in a sealed environment. Move the device to a magnetic stirrer and start stirring, then slowly add 1.35 g of thionyl chloride dropwise in batches. After the addition, heat up and control the temperature at 60°C. After stirring for 3 hours, the reaction is complete, and the solution turns into an oily clear liquid. Stop stirring and stop heating. Take a 350ml beaker and add tap water, the amount added is half of the capacity of the beaker, after the reaction solution is naturally cooled, pour the reaction solution into the beaker, the reaction solution quickly crystallizes into a white solid, then suck the solid by suction filtration, put the solid in an oven to dry , to o...
Embodiment 2
[0040] Take 20ml of methanol into a three-neck bottle, then weigh 3g of 2-chloro-3-methyl-4-methanesulfonylbenzoic acid and add it, then put it into the rotor, add 10ml of methanol, install a reflux device and a thermometer, and The mouth is coated with vacuum ester, so that the whole reaction is carried out in a sealed environment. Move the device to a magnetic stirrer and start stirring, then slowly add concentrated sulfuric acid dropwise in 3 times. The total amount of concentrated sulfuric acid added is 1.35g. After the addition, heat up and control the temperature at 62.5°C. After stirring for 7 hours, the reaction is complete. , the solution completely turns into an oily clear liquid, stop stirring and stop heating. Take a 350ml beaker and add tap water, the amount added is half of the capacity of the beaker. After the reaction solution cools down naturally, pour the reaction solution into the beaker. The reaction solution quickly crystallizes into a white solid, then su...
Embodiment 3
[0045] Take 20ml of methanol into a three-neck bottle, then weigh 3g of 2-chloro-3-methyl-4-methanesulfonylbenzoic acid and pour it into the bottle, then put it into the rotor, add 10ml of methanol, and install a reflux device and a thermometer , the bottle mouth is coated with vacuum ester so that the whole reaction is carried out in a sealed environment. Move the device to a magnetic stirrer and start stirring, then slowly add 1.35 g of concentrated sulfuric acid dropwise in 3 batches. After the addition, heat up and control the temperature at 65°C. After stirring for 7 hours, the reaction is complete, and the solution turns into an oily clear liquid. Stop stirring and stop heating. Take a 350ml beaker and add tap water, the amount added is half of the capacity of the beaker, after the reaction solution is naturally cooled, pour the reaction solution into the beaker, the reaction solution quickly crystallizes into a white solid, then suck the solid by suction filtration, put...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com