Method for detecting furosemide in human plasma by liquid chromatography-mass spectrometry and application thereof
A technique of liquid chromatography-mass spectrometry and furosemide, which is applied in the field of biological analysis, can solve the problems of poor selectivity, long time and time consuming, and achieves the effect of short detection time and good accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0047] The present invention is described below through specific embodiments. Unless otherwise specified, the technical means used in the present invention are methods known to those skilled in the art. In addition, the embodiments should be considered as illustrative rather than limiting the scope of the invention, the spirit and scope of which is defined only by the claims. For those skilled in the art, on the premise of not departing from the spirit and scope of the present invention, various changes or modifications to the material components and dosage in these embodiments also belong to the protection scope of the present invention. The raw materials and reagents used in the present invention are commercially available.
[0048] Materials and Methods
[0049] 1. Instruments and reagents
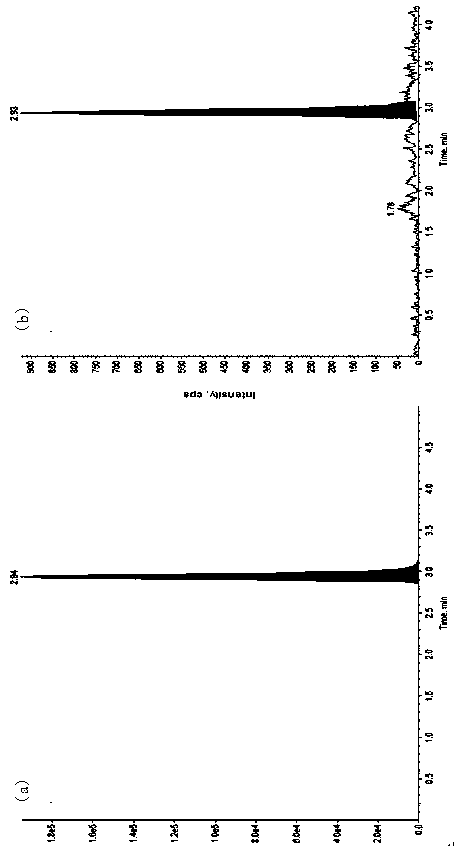
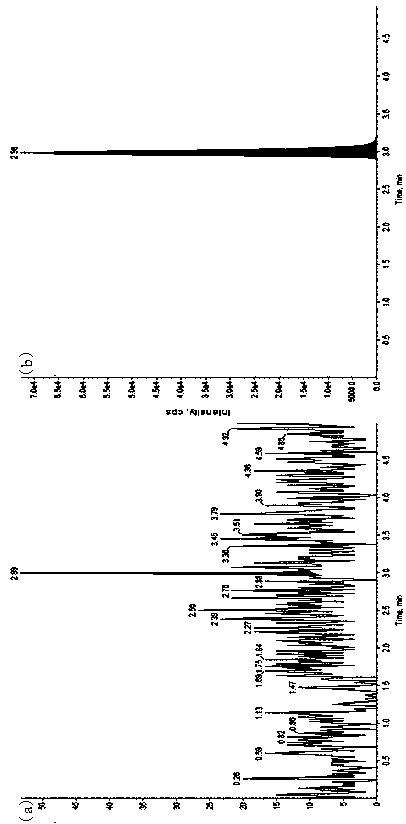
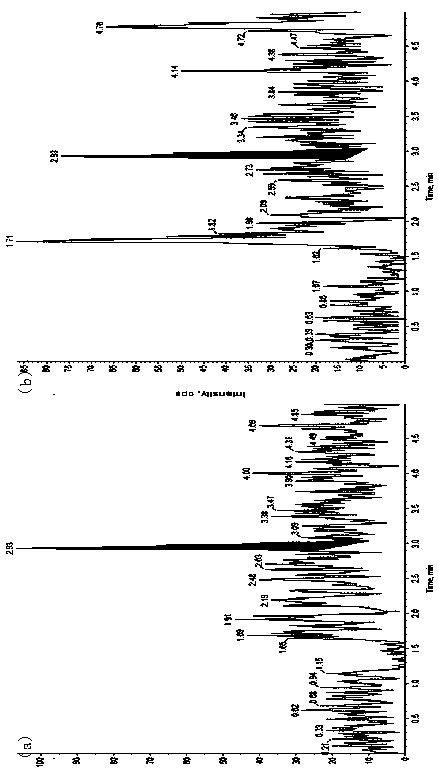
[0050] Ultra-high-phase liquid chromatography (Waters Acquity UPLC); mass spectrometry (API 4000, Applied Biosystems / Sciex); water purifier (MilliDirectQ, Millipore); microbalance (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com