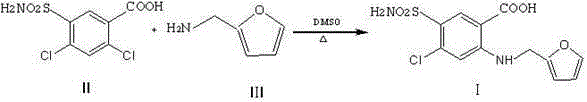
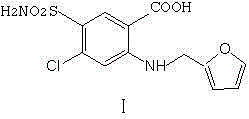
Preparation method of furosemide
A technology of furosemide and furosemide, applied in directions such as organic chemistry, can solve the problems of high synthesis cost, high price, unfavorable industrialized production and the like, and achieve the effects of low cost, convenient operation, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Add 150ml of dimethyl sulfoxide into the reaction flask, then add 81g of 2,4-dichloro-5-sulfonylaminobenzoic acid and 30g of sodium bicarbonate, fill it with nitrogen for three times, and then raise the temperature to the reaction temperature of 130°C. Start to add 87g of furfurylamine dropwise, keep it warm for 6 hours, cool down to 30°C, adjust the pH to 3-4 with 10% hydrochloric acid, cool down to 20°C, keep for 2 hours, precipitate crystals, and filter out to obtain crude furosemide , Put the crude product into a three-necked flask, add 200ml of ethanol, 200ml of water, and 10g of sodium hydroxide, stir and heat up to 70°C to dissolve, then add 5g of activated carbon, filter it after 30 minutes, and cool the filtrate to room temperature, adjust the pH with 15% hydrochloric acid Value, solid precipitated, until PH = 3-4, kept for 2 hours, suction filtered, and the filter cake was dried to obtain 70.5g of furosemide, which was analyzed by HPLC with a purity of ≥99.0%. ...
Embodiment 2
[0018] Add 150ml of N,N-dimethylformamide into the reaction flask, then add 81g of 2,4-dichloro-5-sulfonylaminobenzoic acid and 30g of sodium bicarbonate, replace with nitrogen, and then heat up to the reaction flask At a temperature of 130°C, start to add 87g of furfurylamine dropwise. After dropping, keep it warm for 5 hours, cool down to 30°C, adjust the pH to 3-4 with 10% hydrochloric acid, cool down to 20°C, keep for 2 hours, precipitate crystals, and filter out to obtain For the crude product of furosemide, put the crude product into a three-necked flask, add 200ml of ethanol, 200ml of water, and 12g of sodium hydroxide, stir and heat up to 70°C to dissolve, add 5g of activated carbon, heat filter after 60 minutes, and cool the filtrate to room temperature. Adjust the PH value with % hydrochloric acid, and precipitate a solid until PH = 3-4, keep for 1 hour, filter with suction, and dry the filter cake to obtain 64.2 g of furosemide. After HPLC analysis, the purity is ≥99...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com