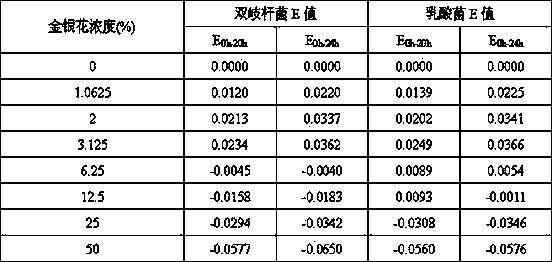
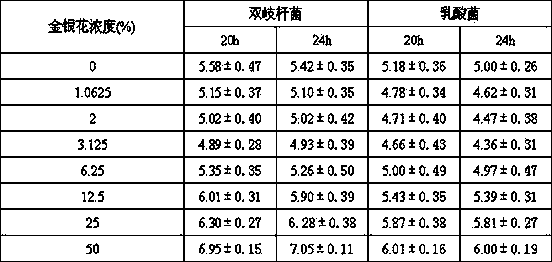
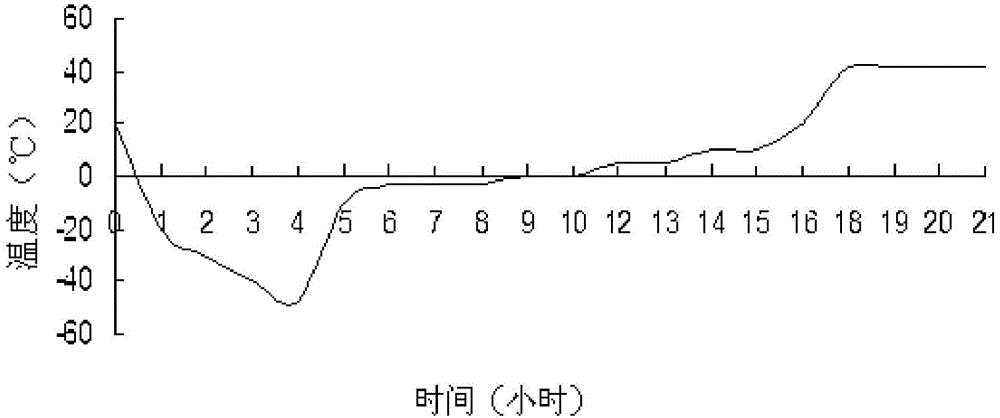
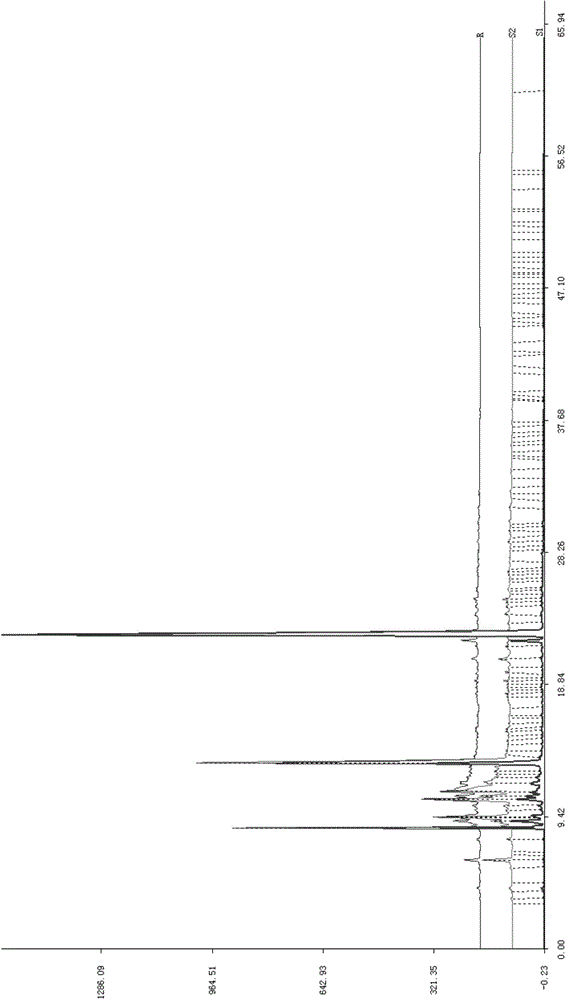
Patents
Literature
85 results about "Pig brain" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
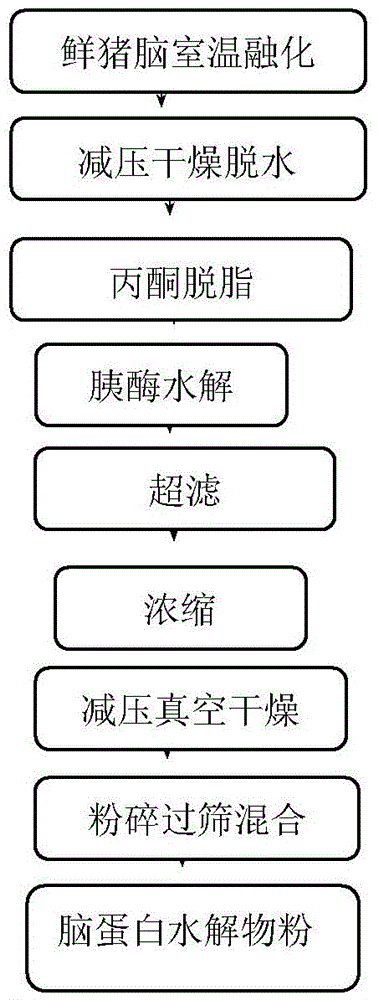
Method for preparing pharmaceutics of hydrolysate of brain protein
ActiveCN1562339ATo achieve the separation effectNo pollution in the processPowder deliveryNervous disorderHydrolysateTyrosine
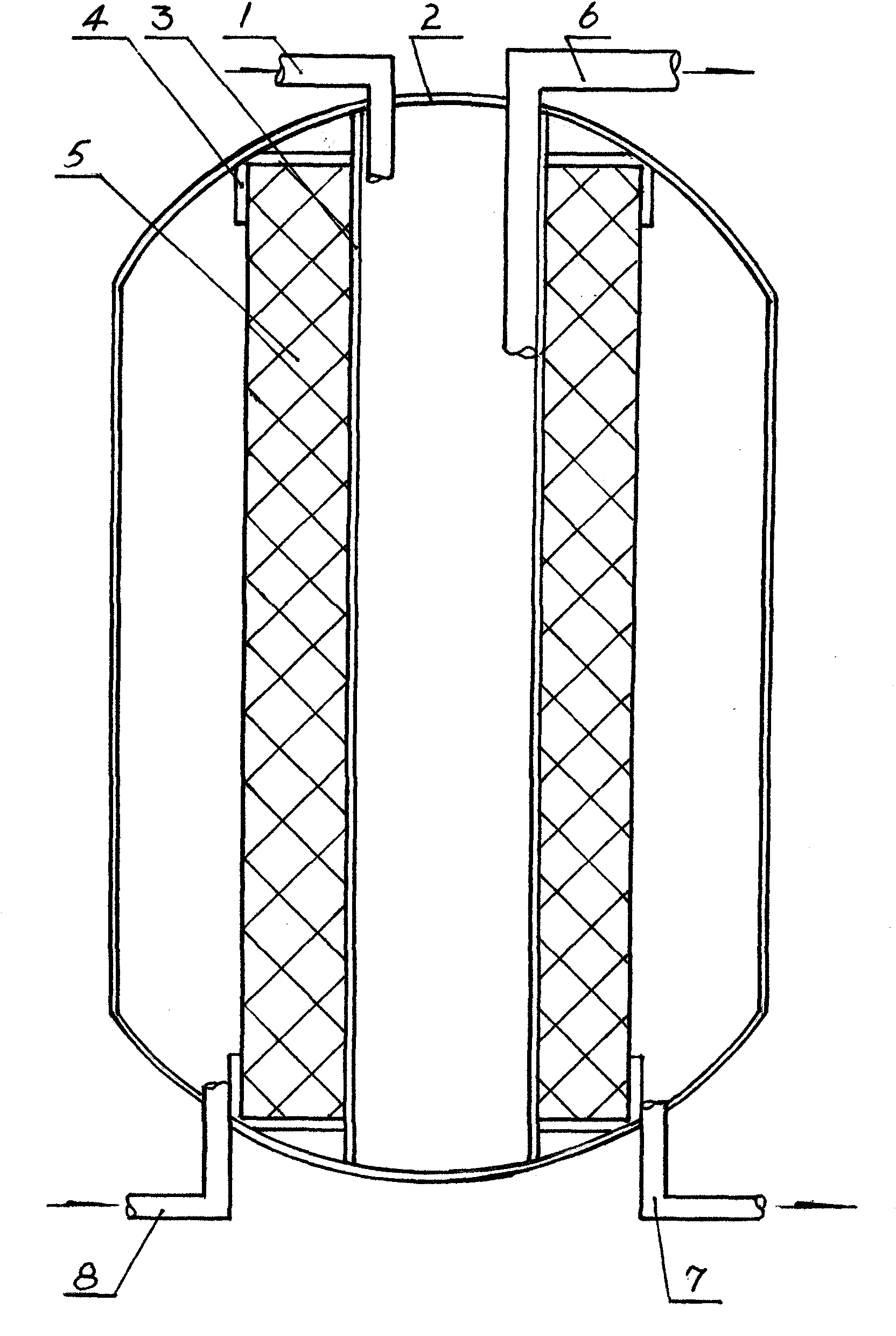
A hydrate of brain protein is prepared from pig's brain through adding purified water, homogenizing, heating, cooling, regulating pH=1.5-2.0, enzymolyzing, regulating pH=7.7-8.0, enzymolyzing, regulating pH=2.5-3.0 freezing, thawing, filter, regulating pH to become neutral, ultrafiltering, concentrating, sterilizing, adding amino acids, regulating peptide map, diluting, and steam sterilizing.
Owner:赛隆药业集团股份有限公司
Brain protein hydrolysate and production process of its freeze dried preparation
InactiveCN1857711ANo pollution in the processReduce investmentPowder deliveryNervous disorderFreeze-dryingNitrogen
The present invention relates to a kind of brain protein hydrolysate and the production process of its freeze dried preparation. The brain protein hydrolysate for injection is prepared with pig brain and through the steps of homogenating in a colloid mill to collect slurry, hydrolyzing with pepsase and pancreatin to collect supernatant, filtering with filer paper to collect filtrate, separating and purifying the filtrate with hydroxyapetite column, regulating peptide map and collecting object, ultrafiltering with membrane of intercepting molecular weight 8 KD to collecting filtrate, nitrogen and amino acid analysis and adding amino acid in the required amount, and fine filtering with 0.22 micron filtering membrane. The brain protein hydrolysate for injection may be further freeze dried to obtain freeze dried brain protein hydrolysate preparation. The production process is environment friendly, high in yield and low in cost.
Owner:HAINAN JINXING PHARMA
Method for preparing brain protein hydrolysate for injection and preparation thereof
The invention relates to a method for preparing brain protein hydrolysate for injection and a preparation thereof. The brain protein hydrolysate is prepared from pig brain tissues passing quarantine through a specific controlled enzyme hydrolysis technique, and active ingredients of the prepared brain protein hydrolysate comprise polypeptide with a molecular weight of less than 10,000 Daltons and 16 free amino acids which are hydrolyzed amino acids; each milligram of total nitrogen of the obtained brain protein hydrolysate contains 4.68 to 7.02 milligrams of amino acids; the preparation method comprises the processes of homogenization, primary enzymolysis, inactivation, secondary enzymolysis, cation exchange column adsorption, desorption, blending, ultra-filtration, preparation and the like; when the obtained brain protein hydrolysate is used for preparing the preparation, an amino acid does not need adding; and pharmacological experiment results show that the prepared preparation has good treatment effect and good safety.
Owner:刘燎原 +1
Coproduction process of pig brain protein hydrolysate and monosialoganglioside
ActiveCN102093440ARealize joint productionImprove utilizationNervous disorderHydrolysed protein ingredientsUltrafiltrationFreeze-drying
The invention discloses a method for obtaining pig brain protein hydrolysate and monosialoganglioside (GM1) from fresh pig brain through fractional extraction. The main process routes are as follows: (1) firstly, adding polar organic solvent homogenate fresh pig brain, and respectively collecting a filter cake and filtrate after filtration; (2) adding a certain amount of low-pole organic solvent and purified water in the filtrate from the step (1) for Folch lamination, then respectively carrying out resin column chromatography, hydrolysis and silicagel column chromatography so as to obtain high pure monosialoganglioside; and (3) adding a certain amount of acetone in the filter cake from the step (1) for degrease, filtering and drying, adding salt buffer solution and hydrolase for hydrolysis, and carrying out inacitivation, ultrafiltration purification, ultrafiltration concentration and vacuum freeze drying so as to obtain medical injection-stage pig brain protein hydrolysate.
Owner:SHANDONG NEWTIME PHARMA
Denatured protein powder and brain protein hydrolyzate prepared from same
ActiveCN102718857AHigh in peptidesQuality assuranceAntibacterial agentsNervous disorderHydrolysateUltrafiltration
The invention relates to a denatured protein powder and a brain protein hydrolyzate prepared from the same. The total nitrogen content of the denatured protein powder is more than 120 mg / g; and the denatured protein powder is prepared by adding fresh lysozyme into a pig brain, homogenating, heating, and defatting with acetone. The denatured protein powder, as a raw material, is used for preparingthe brain protein hydrolyzate; the contents of amino acids in the prepared brain protein hydrolyzate meet national standards of brain protein hydrolyzate injections; and the content of small moleculepeptides with the molecular weight of less than 10000 Da is 25-35% of the brain protein hydrolyzate. The preparation method of the brain protein hydrolyzate mainly comprises the following steps: pre-treating, homogenizing, defatting with the acetone, performing dual enzymatic hydrolysis, centrifugating, refining by column chromatography, and performing ultrafiltration treatment. The invention discloses key technical points for controlling the total nitrogen index of the protein powder and the content of the small molecule peptides for the first time; the obtained brain protein hydrolyzate is a biologically active substance which is really extracted from the pig brain, without adding active components artificially, thus ensuring the efficacy and the safety of the brain protein hydrolyzate;and animal pharmacodynamic experiments show that the brain protein hydrolyzate is more effective than conventional products.
Owner:HEBEI ZHITONG BIOLOGICAL PHARMA
Method for preparing brain polypeptide and brain small-molecule peptide by means of pig brain protein through enzymolysis
ActiveCN105331665AImprove efficiencySimple processPeptide preparation methodsFermentationMicrowaveOrganic solvent
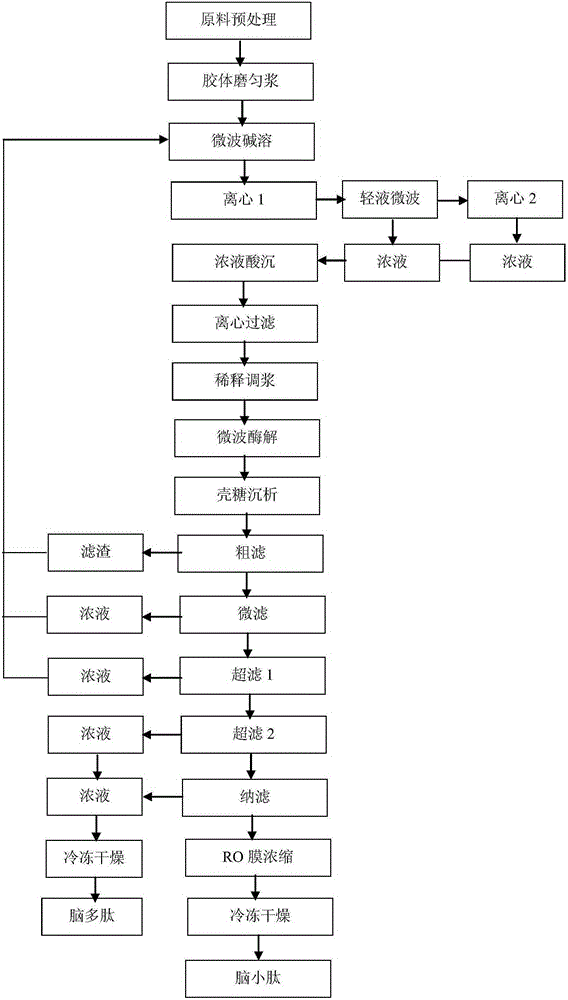
The invention belongs to the technical field of bioengineering, and particularly relates to a method for preparing brain polypeptide and brain small-molecule peptide by means of pig brain protein through enzymolysis. The method for preparing the brain polypeptide and the brain small-molecule peptide by means of the pig brain protein through enzymolysis comprises the following steps of raw material preprocessing, pulp grinding, microwave processing, centrifuging, pulp conditioning, microwave enzymolysis performing, precipitating, rough filtering, micro-filtering, ultra-filtering, nano-filtering, RO membrane concentrating and freeze drying. According to the method, a microwave is in coupling connection with a high-speed centrifuge to replace the method that the pig brain isolate protein is extracted through organic solvent, the technology is simple, the time is short, the efficiency is high, and the production and labor are safe and reliable; the brain isolate protein extraction and enzymolysis time is short, and therefore the production cycle is short; the method is simple in technology, easy to control and suitable for being adopted by factory mass production.
Owner:深圳知本康业有限公司
Method for preparing monostalotetrahexosyl gangliside GM1 compound
InactiveCN101797261AEliminate pollutionImprove permeabilityOrganic active ingredientsNervous disorderPurification methodsSeparation technology
The invention discloses a method for preparing monostalotetrahexosyl gangliside GM1 compound, which belongs to the technical field of the biochemical pharmacy. Combining the separation technologies of hyperfiltration membrane and nanofiltration membrane and on the basis of maintaining the bioactivity and improving the curative effect of gangliside GM1 compound, the gangliside GM1 is extracted and purified from pig brain from the process steps such as homogenization, filter pressing, hyperfiltration, nanofiltration and the like, and the purity of the ganliside GMI reaches more than 95 percent. Relevant steps of the traditional chromatograph and column chromatography purification method are reduced, and the application of harmful organic solvent is reduced. The method has the advantages that the industrialized production of the entire process can be realized, the production cost and the production expense can be reduced, not only bioactivity can be maintained, and the curative effect of gangliside GM1 compound can be improved, but also the preparation process is simple and environment-friendly and is free from the pollution.
Owner:吕维学
Single sialic acid tetrahexose ganglioside preparation method
InactiveCN1799552ASave solventShorten the timeOrganic active ingredientsPowder deliverySolventIntegument
The invention relates to a method for preparing mono sialic acid hexose ganglioside sodium agent, comprising removing integument and blood vessel from the fresh or frozen pig brain or cattle brain, pushing for dewatering with cold acetone for several times, filtering with organic membrane, pouring out the acetone, and treating into acetone powder, packing, and conserving; extracting clarified solution from acetone powder, filtering with ultra-filter membrane and getting ultra-filtrate, extracting with tobacco extract, sodium filtering with sodium filter membrane to remove small molecule, abandoning the filtrate, getting clean condensed liquid of the mono sialic acid hexose ganglioside sodium agent; sterilizing and getting the refine product. The invention changes the polarity of the extracting organic solvent, which makes the ganglioside be released almost fully into the solution. The invention employs the ionic sodium filter membrane to separate and converse the ganglioside depending on the its number difference in sialic acid and carboxyl groups, and employs modern membrane separation technique to replace filling material and column suction technique, which saves large quantity of solvent and time, and increases productivity.
Owner:严家定
Extraction technology of cerebral polypeptides
InactiveCN102863508ACompletely degradedEnhanced Biosecurity ControlsPeptide preparation methodsActive enzymeNucleotide
The invention discloses an extraction technology of cerebral polypeptides, relating to the technical field of biochemical industry. The technology comprises the following steps: taking quarantined fresh pig brains and removing impurities, washing with purified water, then adding 4 times of purified water and conducting high speed cell breakage with a high-speed blender at twice; filtering with a filter cloth, and then homogenating by a homogenizer; adjusting the pH value, hydrolyzing by using complex enzyme to obtain low molecular weight small peptide, a mixture of free amino acid and nucleotide, heating up the enzymatic hydrolysate to 40-50 DEG C for removing active enzymes, distributing the enzyme removal liquid into containers, putting the containers in a refrigerator of -200 DEG C, freezing the containers for 12-24h and then taking the containers out, putting the containers at room temperature for thawing, taking the supernatant and centrifuging the supernatant with a centrifugal machine, removing fat, cell debris, residual denatured protein, nucleic acid and other large molecular impurities, taking the centrifugate and conducting unltrafiltration by using an unltrafiltration device. Compared with traditional process, the technology disclosed herein has the advantages of time saving, high efficiency, safety and environmental protection.
Owner:江西康宝医药生物科技有限公司
Preparation method of ganglioside extract
InactiveCN106349303AFast heatingImprove drying efficiencyNervous disorderSugar derivativesSodium acetateFiltration
A preparation method of ganglioside extract includes subjecting fresh pig brains to microwave vacuum drying treatment to obtain dry pig brains with moisture content lower than 5wt%, and grinding the dry pig brains into pig brain powder, wherein the vacuum degree for microwave vacuum drying ranges from 0.04 Mpa to 0.09 Mpa; adding absolute ethyl alcohol into the dry pig brain powder, stirring for 2.0-5.0 hours at 35-55 DEG C, and filtering to obtain precipitate I; adding a mixed solvent of an acetic acid-sodium acetate buffer solution with a pH being 5.0-5.4, isopropanol and dichloromethane into the precipitate I, stirring for 60-120 minutes at a room temperature, and conducting centrifugal separation to obtain liquid supernatant I; cooling the liquid supernatant I to 15-25 DEG C to enable precipitate II to separate out, and filtering to obtain the precipitate II; repeatedly washing the precipitate II by anhydrous ether prior to filtration and vacuum drying so as to obtain the ganglioside extract. The preparation method of the ganglioside extract has the advantages that consumption of harmful solvents is reduced substantially, and the prepared ganglioside extract is high in ganglioside purity and GM1 content.
Owner:丁海麦
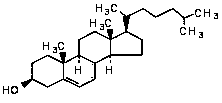
Cholesterol extraction process
The invention discloses a cholesterol extraction process including the following operations: (1) preparing a pig brain homogenate from a pig brain; (2) adding an extraction solvent into the pig brainhomogenate to obtain an extraction solution; (3) filtering the extraction solution, concentrating the filtered liquid to obtain a concentrated solution, and recovering the solvent; (4) adding a methanol solution to the concentrated solution, filtering a refluxed liquid and crystallizing for 12-72 h, to obtain crystals; (5) filtering the crystals in the step (4), adding methanol with the amount 4-7times of the mass of the crystals, refluxing for 1 h at the temperature of 70 DEG C, placing the refluxed liquid at the temperature of 0 DEG C, and crystallizing for 12-72 h; (6) filtering the crystals in the step (5), adding methanol with the amount 2-5 times of the mass of the crystals with the temperature of methanol of -30-0 DEG C, and washing to be neutral; (7) adding methanol with the amount 3.5-6 times of the mass of the neutral crystals of the step (6), and refluxing for 1 h at the temperature of 70 DEG C; and placing the refluxed solution at the temperature of 0 DEG C, crystallizingfor 12-72 h, and filtering the crystals; and (8) carrying out vacuum drying of the final crystals of the step (7) at the temperature of 70-80 DEG C, to obtain cholesterol. The method has high yield and high content.
Owner:四川奇格曼药业有限公司
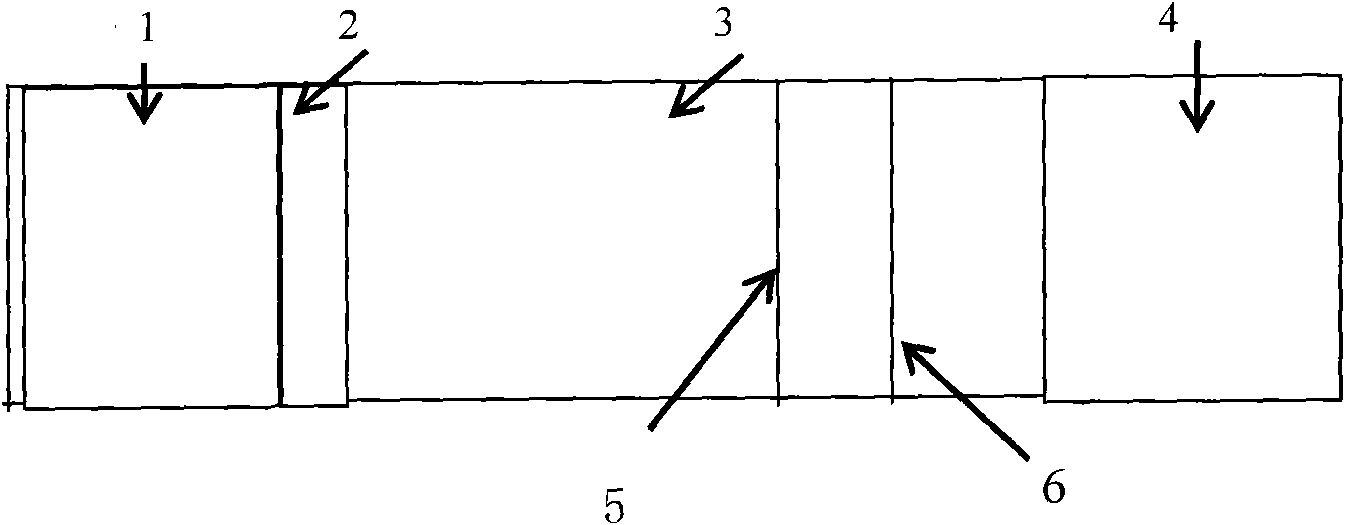
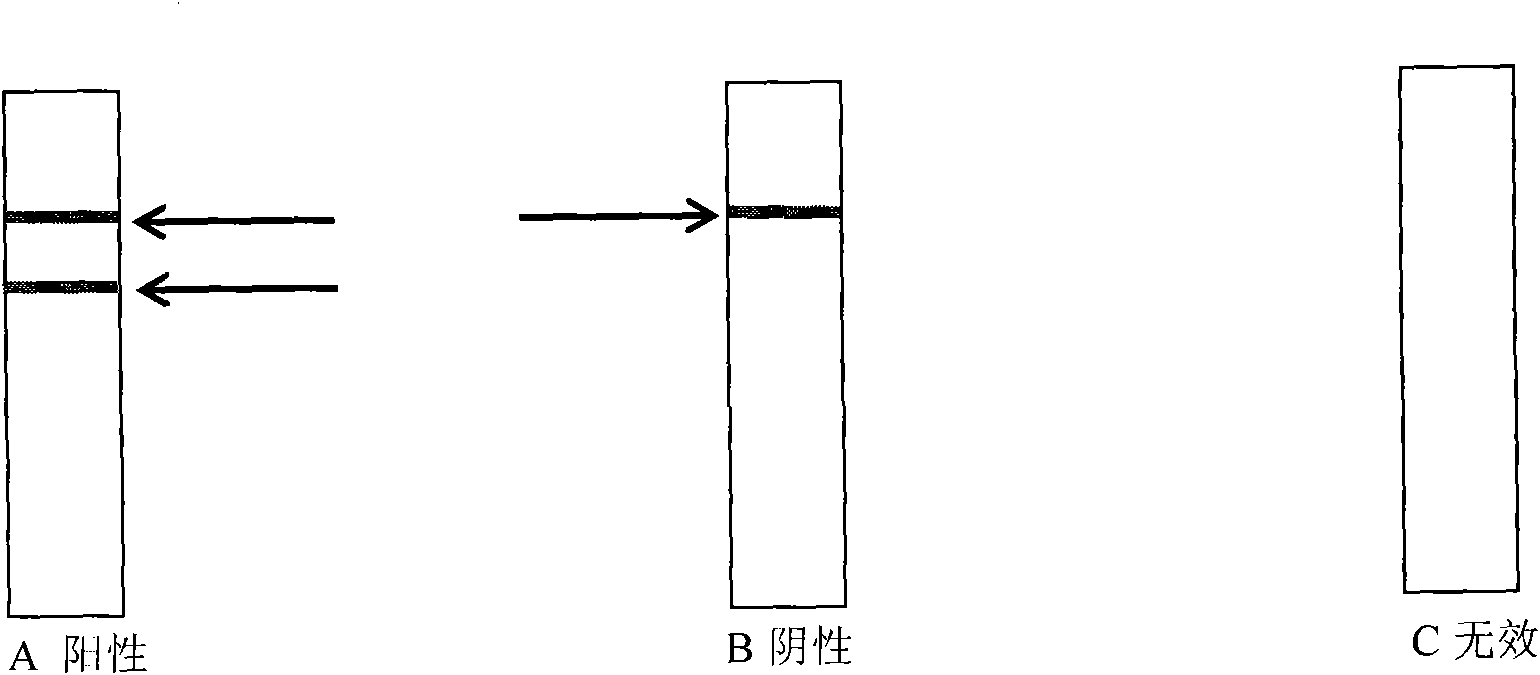
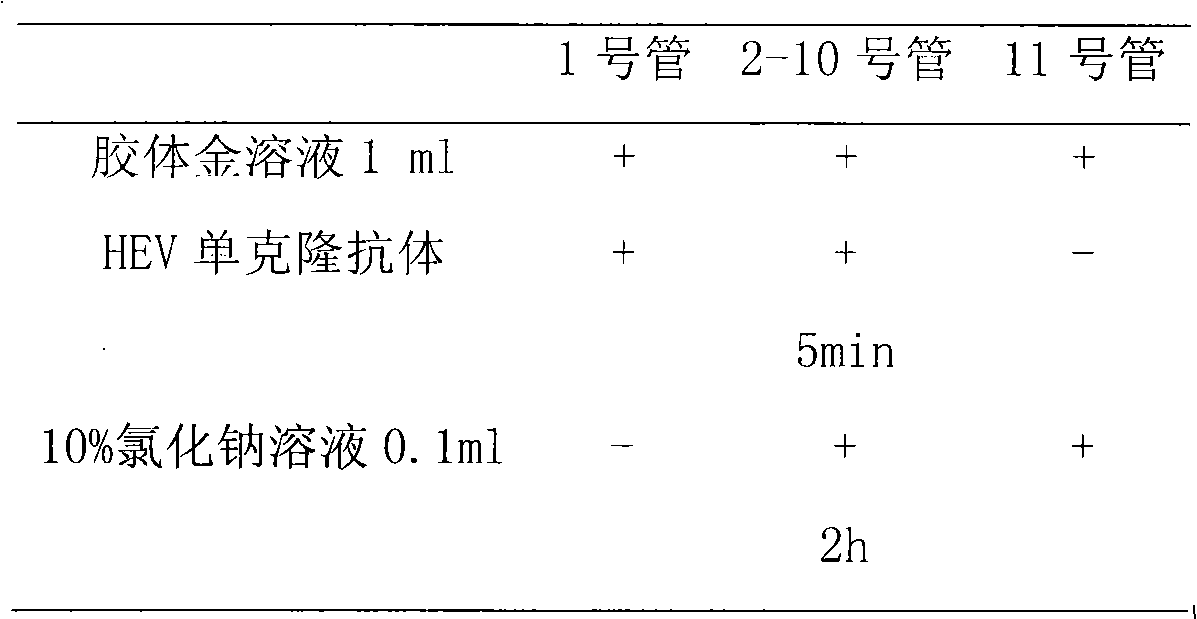
Swine Hemagglutinating encephalomyelitis virus colloidal gold antigen detecting test paper and preparing method thereof
The present invention relates to a novel technique-colloidal gold immunochromatography for quickly detecting the Swine Hemagglutinating encephalomyelitis virus (HEV), and to a test paper which is prepared by the technique for preparing the Swine Hemagglutinating encephalomyelitis virus. The invention develops a monoclonal antibody (Ab1) of the Swine Hemagglutinating encephalomyelitis virus and a monoclonal antibody (Ab2) of rabbit anti- Swine Hemagglutinating encephalomyelitis virus. The Ab1 labeled colloidal gold is taken as the gold-labeled antibody and the Ab2 is taken as the catching antibody. The normal anti-mouse IgG antibody is used as a quality control antibody. The materials of antibody, cellulose nitrate film (NC film), combining pad, sample pad, back lining, plastic card, etc. are processed and assembled to the test paper. The test paper can detect the Swine Hemagglutinating encephalomyelitis virus in the sickened pig brain tissue and the feces. The technique of the invention has the characteristics of simple operation, high speed, excellent sensitivity, excellent specificity, etc. Furthermore the technique of the invention is especially suitable for the clinic sample detection of disease appearance field, clinic, places with no available experiment condition, etc.
Owner:JILIN UNIV
Multi-biological activity function food and preparation process thereof
InactiveCN103989041AImprove defense functionLow priceNatural extract food ingredientsFood ingredient functionsDiseaseLicorice roots
The present invention discloses a multi-biological activity function food, which is characterized in that 270 kg of the food comprises 1-3 kg of pig brain / pig spleen polypeptide, 6-16 kg of a Chinese herb extract, 180-210 kg of oligomeric polysaccharide, and the balance of water and trace elements, wherein the Chinese herb extract is one or a plurality of materials selected from a mixed water extract of honeysuckle, hawthorn and licorice root, a pericarpium citri reticulatae water extract, a medlar water extract and a lotus seed water extract. The preparation process comprises: preparing a Chinese herb extract and pig brain / pig spleen polypeptide, weighing various components according to the ratio, uniformly mixing, and carrying out sterilization and packaging. According to the present invention, the synergy effect of the transfer factor, the oligomeric polysaccharide and the traditional Chinese medicines with medical and edible dual-purpose in many aspects are comprehensively utilized, and the effects of human body defense function enhancing, circadian rhythm regulating, disease preventing and rehabilitation promoting are provided.
Owner:NANCHANG UNIV
Pig brain extract, composition containing pig brain extract and application of composition
ActiveCN105998072ASimple methodReduce errorsOrganic active ingredientsNervous disorderPhysiologyGamma-Aminobutyric acid
The invention relates to the technical field of biological medicine, in particular to a pig brain extract, a composition containing the pig brain extract and application of the composition. The molecular weight of the pig brain extract is no more than 10 kDa and no less than 200 kDa, and the pig brain extract mainly comprises micro-molecule polypeptides or free amino acid, cephalin, lecithin, peptide nerve growth factors and the like. Experiments show that the composition obtained by compounding the pig brain extract, gamma-aminobutyric acid and linseed oil powder can achieve the effects of strengthening the brain and reinforcing intelligence.
Owner:HANGZHOU HUAJIN PHARMA
Production technology of pig brain protolysate
InactiveCN101524370AHigh degreasing rateIncrease the degree of hydrolysisNervous disorderUnknown materialsAnimal scienceHydrolysate
The invention provides a production technology of pig brain protolysate. The technology comprises two main courses: (1) a fresh and healthy pig brain is subjected to procedures like decreasing to obtain decreased brain powders, (2) the decreased brain powders are subjected to procedures like hydrolysis to obtain the product. Compared with the prior art, the invention simplifies the technologic processes, changes the current secondary decreasing method into the dual-degreaser primary decreasing method, and improves the decreasing rate to further strengthen the degree of the hydrolysis; the mixture, which is obtained by homogenating inexpensive, fresh and healthy pig pancreas and fresh pineapple peels, is used for replacing high-priced commercial enzyme used for hydrolysis. Production cost is reduced while the quality of the product is guaranteed.
Owner:NORTHEAST FORESTRY UNIVERSITY
Beauty and lipid-lowering health flower tea and preparation method thereof
InactiveCN104304593APromote blood circulationPromote circulationTea substituesTremellaLipid lowering
The invention discloses a beauty and lipid-lowering health flower tea and a preparation method thereof. The flower tea consists of the following raw materials in parts by weight: 100-110 parts of rose, 100-110 parts of apple blossom, 7-9 parts of pig brain, 18-20 parts of chitterlings, 4-6 parts of tremella, 15-18 parts of watermelon peel juice, 9-11 parts of chocolate juice, 6-7 parts of fresh cervus elaphus Linnaeus, 3-5 parts of pickled beans, 4-5 parts of preserved duck eggs, 9-10 parts of passion flower, 4-5 parts of sugar palm powder, 1-2 parts of hawthorn, 1-2 parts of Polygonum multiflorum, 1-2 parts of cassia seeds, 1-2 parts of cortex albiziae, 1-2 parts of lotus leaves, an appropriate amount of water and 15-20 parts of nutritional additives. The flower tea uses rose and apple blossom as main materials, and has the effects of nourishing liver and appetizing, promoting qi to activate blood, maintaining beauty and keeping young, making people exhilarating, promoting Qi, activating blood, converging, alleviating neuralgia, replenishing blood and improving eyesight, removing acnes and whitening; added traditional Chinese medicine has the effects of reducing blood fat and softening blood vessels; the flower tea can be used for enhancing blood circulation of coronary artery, and is resistant to arrhythmia and hyperlipidemia.
Owner:吴秀杰
Method for rapidly and efficiently preparing high-purity ganglioside
InactiveCN108546276AImprove separation efficiencyEfficient separationSugar derivativesSugar derivatives preparationFiltrationHigh pressure
The invention relates to the field of biopharmaceuticals, in particular to a method for rapidly and efficiently preparing high-purity ganglioside. The method comprises the following specific steps ofextraction of a pig brain extract, GM1 silica gel chromatography purification and cationic resin purification, wherein the extraction of the pig brain extract comprises the following steps of extraction, stratification, saponification, resin chromatography, acid hydrolysis, acetone precipitation filtration and drying. Through extraction of the ganglioside with a heat extraction method, the extraction efficiency is improved greatly. The GM1 content is increased by more than twice through acid hydrolysis. Moreover, a high-pressure system is used for performing silica gel purification, thereby greatly shortening the purification time while improving the separation efficiency. Rapid and effective separation of GM1 and impurities is realized, on-line detection can be realized, subsequent point-plate detection is not needed, and the production efficiency is greatly improved. Moreover, substances affecting the clarity of an injection are removed through cationic resin chromatography, so thatthe occurrence rate of adverse reactions after treatment is reduced greatly.
Owner:海南益尔生物制药有限公司
Cerebroprotein hydrolysate freeze-dried powder injection and preparation method thereof
ActiveCN104096214AInhibition of recombinationPrevent reversible bindingPowder deliveryNervous disorderFreeze-dryingFiltration
The invention provides a cerebroprotein hydrolysate freeze-dried powder injection and a preparation method thereof. The preparation method includes: taking a pig brain, removing impurities, adding purified water to conduct homogenization; subjecting the obtained homogenate to hydrolysis by pepsin and pancreatin successively, and collecting the clear liquid; adjusting the pH of the clear liquid to 1.7-3, conducting heating to 100-110DEG C and performing heat preservation for 15-45min, and then adjusting the pH to 8.0-9.0; carrying out ultrafiltration by a 8-10kd membrane, and collecting the filtrate; subjecting the filtrate to column chromatography, adjusting the pH of the eluent to 8.0-9.0, performing concentration by an anion exchange membrane, and conducting filtering by a membrane of 0.2 micrometer to obtain a stock solution; freezing the stock solution, then performing thawing, adding an excipient, adjusting the pH to 6.9-7.5, carrying out 8-10kd membrane ultrafiltration, and performing filtration by a 0.2 micrometer membrane; and carrying out filling and freeze-drying. In the obtained freeze-dried powder injection, the similarity of polypeptide and a reference substance is greater than or equal to 0.90, and the freeze-dried powder injection contains 16 amino acids, the nitrogen content of total amino acids accounts for greater than or equal to 85% of the total nitrogen content, and no additional amino acids are needed. The cerebroprotein hydrolysate freeze-dried powder injection is prepared entirely by pig brain hydrolysis, and is stable and safe.
Owner:GUANGZHOU YIPINHONG PHARMA +4
Method for preparing pharmaceutics of hydrolysate of brain protein
ActiveCN1228082CTo achieve the separation effectNo pollution in the processPowder deliveryNervous disorderHydrolysateTyrosine
A hydrate of brain protein is prepared from pig's brain through adding purified water, homogenizing, heating, cooling, regulating pH=1.5-2.0, enzymolyzing, regulating pH=7.7-8.0, enzymolyzing, regulating pH=2.5-3.0 freezing, thawing, filter, regulating pH to become neutral, ultrafiltering, concentrating, sterilizing, adding amino acids, regulating peptide map, diluting, and steam sterilizing.
Owner:赛隆药业集团股份有限公司
Preparation process of high-quality sodium monosialotetrahexosylganglioside
InactiveCN108822164AHigh purityEfficient removalSugar derivativesSugar derivatives preparationActivated carbon filtrationFiltration
The invention relates to the field of drug preparation and in particular relates to a preparation process of high-quality sodium monosialotetrahexosylganglioside. The preparation process comprises a pig brain crude extraction step, a silica gel chromatographic purification step, an activated carbon filtration step and an anion resin chromatography step, wherein the pig brain crude extraction stepcomprises an extraction step, a layering step, a saponification step, a resin chromatography step and a concentration, filtration and drying step. Sodium monosialotetrahexosylganglioside prepared by applying the process is high in purity, heat source substances can be effectively removed by activated carbon treatment, and protein substances capable of resulting in allergy can be effectively removed by anion resin chromatography, so that the purity can be further increased, and the occurrence rate of adverse reactions is obviously reduced.
Owner:海南益尔生物制药有限公司
Brain protein hydrolysate preparation method
InactiveCN105396119AAvoid strong basesLoose structureNervous disorderHydrolysed protein ingredientsChemical industryBrain Cell
The present invention belongs to the technical field of chemical industry pharmacy, and specifically relates to a brain protein hydrolysate preparation method, which comprises: soaking pre-treated and dried pig brain 2-3 times with acetone, defatting, and drying; and hydrolyzing with trypsin, separating the enzyme hydrolyzing mixing liquid after the hydrolyzing so as to collect the supernatant, carrying out purification concentration on the supernatant, and drying to obtain the brain protein hydrolysate. According to the present invention, the extract has characteristics of brain cell nourishing and brain capillary dredging, achieves functions of brain activity restoring, brain cell function improving and memory increasing, and can completely improve hypomnesis and other brain atrophy symptoms; and the organic solvent consumption during the defatting process of the method of the present invention is reduced, the prepared brain protein hydrolysate is the powder, has low viscosity, and is suitable for the subsequent production of the brain protein hydrolysate tablet preparation, and the content is higher than the existing technical standard.
Owner:卫材(辽宁)制药有限公司
Feed for mutton sheep in adult stage
InactiveCN108185169AImprove disease resistanceIncrease release rateFood processingAnimal feeding stuffGaining weightAdult stage
The invention discloses a feed for mutton sheep in adult stage, and relates to the technical field of sheep culturing and breeding. The feed is characterized by comprising the following materials in parts by weight: material 1: 8 to 13 parts of corn straw, 3 to 8 parts of black beans, 3 to 7 parts of wheat straw, 4 to 10 parts of soybean meal, 5 to 10 parts of black rice, 1 to 4 parts of glutinousrice, 1 to 5 parts of bagasse, 1 to 3 parts of roses and 5 to 10 parts of peanut kernels; material 2: 1-5 parts of abalones, 1-5 parts of shark fins, 2-7 parts of kelp, 1-5 parts of chicken livers, 1-4 parts of duck hearts, 1-3 parts of sea horses, 1-5 parts of squids and 1-4 parts of pig brain; material 3: 1 to 4 parts of carrots, 2 to 7 parts of apples, 3 to 8 parts of starfruits, 3 to 8 partsof pomegranates, 4 to 9 parts of grapes, 1 to 4 parts of balsam pears, 1 to 3 parts of laver, 1 to 4 parts of oranges and 4 to 9 parts of dragon fruits; and material 4: 10-15 parts of an auxiliary agent. The feed is reasonable in method and rich in nutrients, and can help gain weight fast.
Owner:ANHUI PROVINCE GUZHEN COUNTY ZHENGHUA SHEEP IND
Sodium sulfadiazine compound preparation for treating pig brain hydropsy, and preparation method and application thereof
InactiveCN102580096APrescription is scientific and reasonableExcipient safetyAntibacterial agentsHydroxy compound active ingredientsMANNITOL/SORBITOLSulfadiazine
The invention discloses a sodium sulfadiazine compound preparation for treating pig brain hydropsy, and a preparation method and application thereof, and belongs to the technical field of veterinary medicines. The sodium sulfadiazine compound preparation comprises sodium sulfadiazine, mannitol, furosemide, trimethoprim and the like. The sodium sulfadiazine compound preparation is scientific and reasonable in formula, safe in auxiliary material, controllable in quality, high in stability and low in cost, and a preparation process is simple and is suitable for industrial production.
Owner:河南省针剂兽药工程技术研究中心
Cerebroprotein hydrolysate and lyophilized powder thereof for injection
ActiveCN103142991AHigh content of active ingredientsGood treatment effectPowder deliveryNervous disorderVacuum pumpingActivated carbon
The invention discloses a cerebroprotein hydrolysate and a preparation method thereof. The extract is prepared by adopting the following method: (1) taking fresh and healthy pig brains, removing fat, homogenizing, then performing vacuum-pumping till 5-10Pa, keeping the pressure for 5-15 minutes, then introducing carbon dioxide until the pressure is 10-18MPa, and keeping the pressure for 20-25 minutes; (2) slowly adding the defatted brains with the saturated carbon dioxide into liquid carbon dioxide in the volume which is 5-8 times of the weight of the defatted brains, stirring for 0.5-2 hours after the end of adding, cooling down to minus 70-minus 80 DEG C, keeping for 2-4 hours, and then increasing the temperature to 40-50 DEG C within 5-10 minutes to gasify the carbon dioxide; (3) adding water for injection in the weight of 2-4 times of that of the frozen-thawed brains obtained in the step (2), uniformly stirring, further adding papain or pepsin which is equivalent to 0.5-1% of the weight of the frozen-thawed brains, and performing hydrolysis at the temperature of 30-40 DEG C for 3-4 hours to get an enzymatic hydrolysate; and (4) centrifugating the enzymatic hydrolysate, taking a supernatant liquid, performing activated carbon adsorption, performing decarbonization and filtering.
Owner:SHANXI PUDE PHARMA CO LTD
Preparation method of uniform and stable type liquid crystal microcapsule material
InactiveCN108786676AImprove stabilityReduce uneven dispersion performanceLiquid crystal compositionsMicroballoon preparationCholesterolIsophorone diisocyanate
The invention relates to a preparation method of a uniform and stable type liquid crystal microcapsule material, and belongs to the technical field of a liquid crystal material. The preparation methodhas the technical scheme that cholesterol ester materials are prepared from pig brain raw materials; the raw materials can be easily obtained; the price is cheap; mixed liquid crystals most show bright colors; meanwhile, IPDI (isophorone diisocyanate), liquid crystals and an organic solvent form O / W emulsion under the effect of emulsifying agents; along with the heating and the addition of catalysts, isocyanate groups in the IPDI and hydroxy of water react to generate amidogen in the IPDI; then, the amidogen and the isocyanate groups take polymerization reaction on an oil-water two-phase interface; a wall urea coated cholesteric phase liquid crystal microcapsule is generated. The stable grafting coating is formed, the dispersion nonuniformity performance among materials is effectively reduced; along with the environment change in the use process, the liquid crystal microcapsule material migrates from inside to the surface; stable bonding and coating modification are formed; the stableperformance of the liquid crystal microcapsule material is greatly improved.
Owner:FOSHAN LINGCHAO NEW MATERIAL CO LTD
Sodium monosialotetrahexosylganglioside preparation method
InactiveCN106905387AHigh purityChromatography works wellSugar derivativesSugar derivatives preparationState of artFiltration
The invention discloses a sodium monosialotetrahexosylganglioside preparation method, which comprises: stirring a raw material such as fresh pig brain to obtain a fresh pig brain concentrated liquid; carrying out resin chromatography on the prepared concentrated liquid, and carrying out suction filtration drying to obtain concentrated powder; dissolving the concentrated powder in a mobile phase, carrying out silica gel chromatography, and carrying out precipitation and drying to obtain crude product concentrated powder; and dissolving the crude product concentrated powder in a chromatographic buffer liquid, carrying out silica gel chromatography, and carrying out precipitation and drying to obtain fine product concentrated powder. According to the present invention, the purification step is divided into the crude purification step and the fine purification step, such that the purity of the prepared sodium monosialotetrahexosylganglioside is high compared to the method in the prior art; and the resin used in the resin chromatography step is subjected to the specific acid and alkali treatment and the cleaning process, and the resin chromatography column is subjected to the strengthening regeneration treatment.
Owner:四川奇格曼药业有限公司
Cerebrolysin hydrolysate injection and preparation method thereof
ActiveCN104043099AAdvantages of preparation processInhibition of recombinationNervous disorderHydrolysed protein ingredientsAntioxidantHydrolysate
The invention provides a cerebrolysin hydrolysate injection and a preparation method thereof. The preparation method comprises the following steps: taking pig brain, removing impurities, and adding purified water for homogenizing; hydrolyzing homogenate through pepsin and pancreatin, and collecting a clear liquid; regulating the pH of the clear liquid to be 1.7-3, heating to 100-110 DEG C, insulating for 15-45min, then regulating pH to 8.0-9.0; conducting ultrafiltration through a 8-10kd membrane, and collecting filtrate; conducting column chromatography to the filtrate, collecting an eluent, regulating ph to 8.0-9.0, concentrating through an anion-exchange membrane, filtering through a 0.2mu m membrane to obtain a stock liquid; and freezing the stock liquid, then defreezing, adding an antioxidant, regulating the pH to be 6.9-7.5, and filtering respectively through a 8-10kd membrane and a 0.2mu m membrane. The polypeptide in the obtained cerebrolysin hydrolysate injection is similar to that of a control drug being more than or equal to 0.90, and the obtained cerebrolysin hydrolysate injection contains 16 amino acids, the nitrogen amount of total amino acids accounts for more than or equal to 85% of total nitrogen content, additional amino acids are not required, and the amino acids can be prepared completely by means of pig brain, thus being stable and safe.
Owner:GUANGZHOU YIPINHONG PHARMA +4
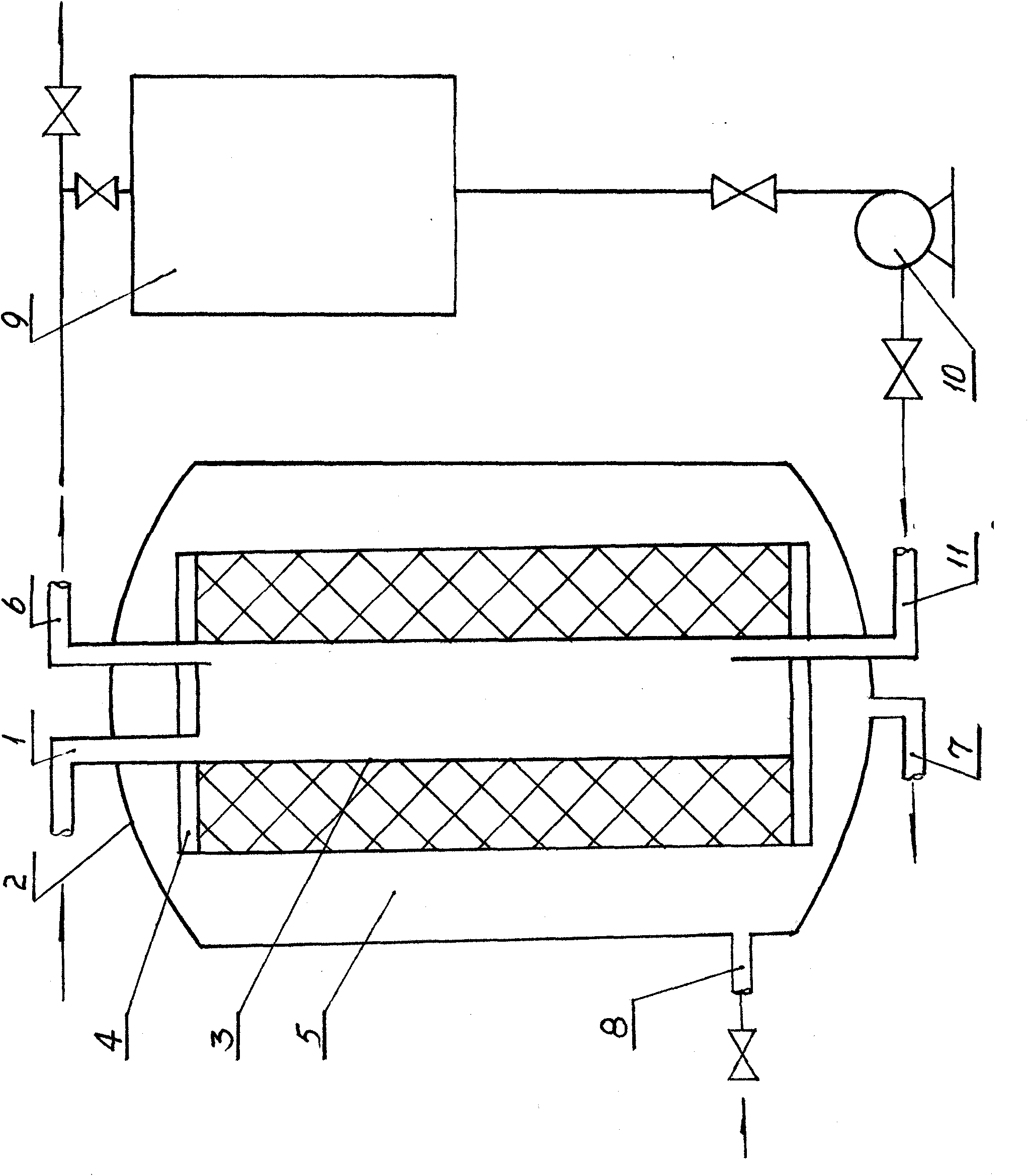
Preparation method and ultrafiltration apparatus of monosialic acid tetrahexose ganglioside GM1 preparation
InactiveCN103788144AEliminate pollutionImprove permeabilitySugar derivativesUltrafiltrationPurification methodsUltrafiltration
The invention discloses a preparation method and an ultrafiltration apparatus of a monosialic acid tetrahexose ganglioside GM1 preparation, which belongs to the technical field of biochemistry pharmacy. The preparation method combines a separating technology of ultrafilter membrane and nanofiltration membrane, from the basis that the biological activity is kept and the curative effect of the ganglioside GM1 preparation is increased, the ganglioside GM1 is extracted and purified from pig brain through the technology steps of homogenizing, filter pressing, ultrafiltrating, nanofiltrating and the like, and the purity of ganglioside GM1 can reach more than 95%. According to the invention, relative links of a traditional chromatography and column chromatography purification method can be reduced, harmful organic solvent usage is reduced, so that the preparation method and ultrafiltration apparatus is suitable for whole process industrial production, production cost and production cost are reduced, biology activity can be kept, the curative effect of the ganglioside GM1 preparation is increased, the preparation process is simple, environmentally friendly and pollution free, and the preparation method and ultrafiltration apparatus have outstanding substantive characteristics and obvious improvement.
Owner:吕维学
Preparing technology for active nano-selenium brain peptide
InactiveCN105154510AAvoid pollutionGuaranteed stabilityPeptide preparation methodsFermentationDiseaseHydrolysis
The invention relates to a preparing technology for active nano-selenium brain peptide. The preparing technology includes the following steps that 1, pig brain tissues are homogenized, enzymolysis degreasing is carried out on the homogenized pig brain tissues through lipase, hydrolysis is carried out through complex enzymes, and ultra-filtration is carried out to obtain brain peptide liquid, wherein the quantity of the added lipase is 0.05% to 0.15% of the weight of brain liquid, and the quantity of the added complex enzymes is 0.2% to 2% of the weight of degreased brain protein liquid; 2, a sodium selenite solution and an ascorbic acid solution are added into the brain peptide liquid, stirring reacting is carried out for 1 hour to 2 hours at the temperature of 40 DEG C to 55 DEG C, and reaction liquid is obtained; 3, spraying drying is carried out on the reaction liquid, and the finished product is obtained. According to the preparing technology, brain protein hydrolysate is degreased in the enzymolysis mode, the degreasing effect is good, environment pollution is avoided, and the biological activity of small molecule peptide and the safety of the product are guaranteed; meanwhile, the small molecule brain peptide obtained after enzymolysis serves as a medium template to adsorb and stabilize nano-selenium, the active brain peptide is combined with the nano-selenium, the physiological functions of the brain peptide and the physiological functions of the nano-selenium are promoted and synergistically interact with each other, and the obtained product has the better treatment effect on various diseases such as insomnia, cephalalgia and hypomnesis.
Owner:陈石良
Chinese medicine for curing coryza
InactiveCN101254268AUnique formulaReasonable formulaUnknown materialsRespiratory disorderBudAdemetionine
The invention discloses a traditional Chinese medicine for the treatment of rhinitis, and comprises the herbal medicines with the following mixing ratio by weight: 50 to 70g of pig brain, 25 to 35g of raw cypress leaf, 8 to 12g of Chinese thorowax root, 5 to 15g of Szechuan lovage rhizome, 10 to 20g of yulan magnolia flower bud, 5 to 15g of dahurian angelica root, 25 to 35g of yellow flower, 6 to 12g of mulberry leaf, 4 to 8g of chrysanthemum, 6 to 12g of sweet apricot seed, 1 to 1.5g of towel gourd stem and 50 to 70g of honey sugar; the invention is proved to have significant efficacy for acute and chronic rhinitis, as well as allergic rhinitis by clinical treatment trials of 200 cases of patients, the cured patients are 190 cases, the cure rate achieves 95 percent; the traditional Chinese medicine can not only carry out the treatment of the symptoms of rhinitis, but can also improve the overall immunity of the human body, thus achieving the purpose of treating both manifestation and root cause of diseases; the treatment time is short, one course of treatment is 7 days, the traditional Chinese medicine can be effective by only one course of treatment, the rhinitis can be cured by two to three courses of treatment, and the rhinitis can not be relapsed after the cure.
Owner:郭天民
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com