Brain protein hydrolysate and production process of its freeze dried preparation
A technology of cerebroprotein hydrolyzate and production method, which is applied in the production field of cerebroprotein hydrolyzate freeze-dried preparations, can solve problems such as long production cycle, susceptibility to microbial contamination, difficult drug quality, etc., and achieve lower production environment requirements and less investment Production equipment, the effect of reducing microbial contamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
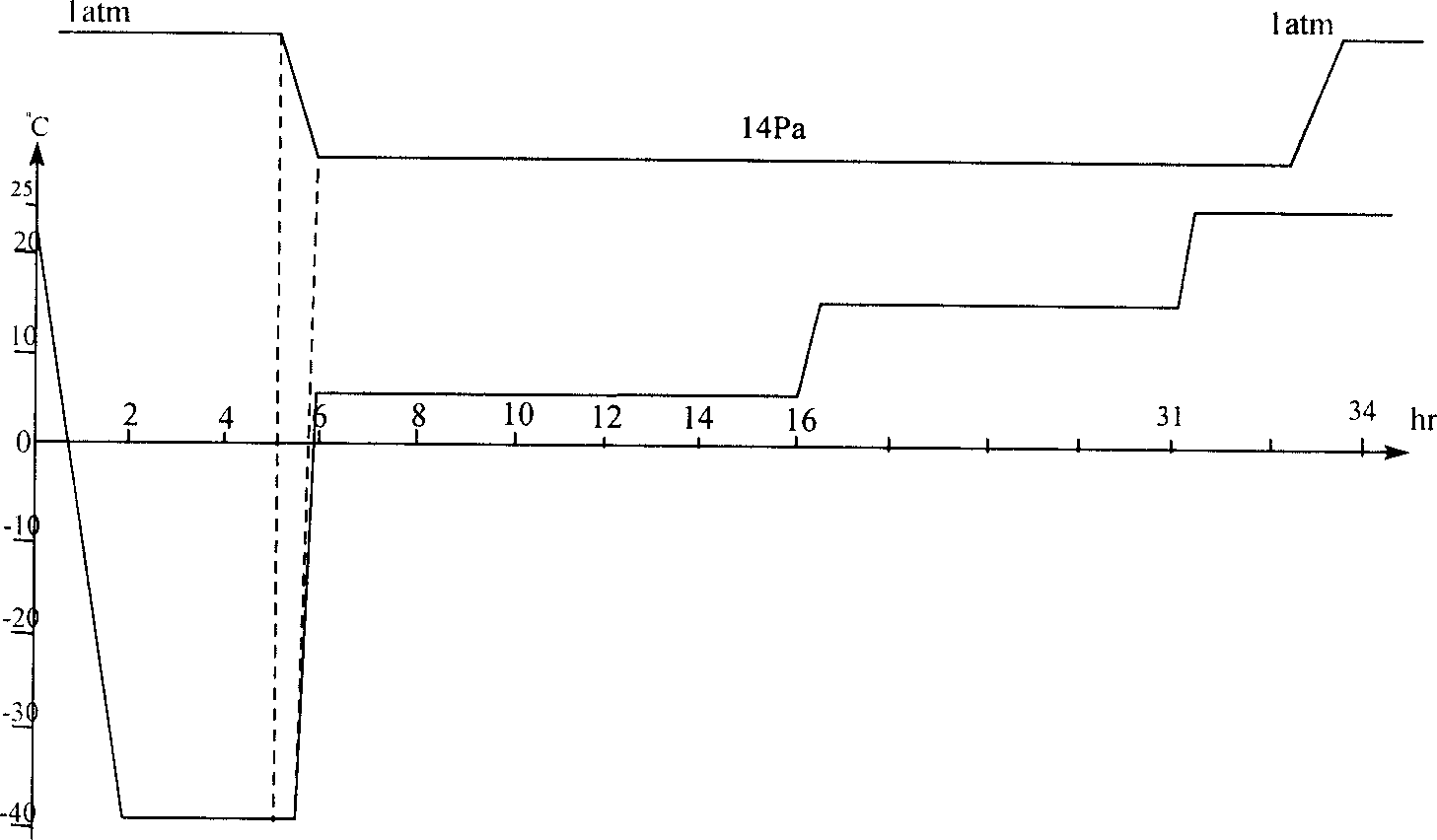
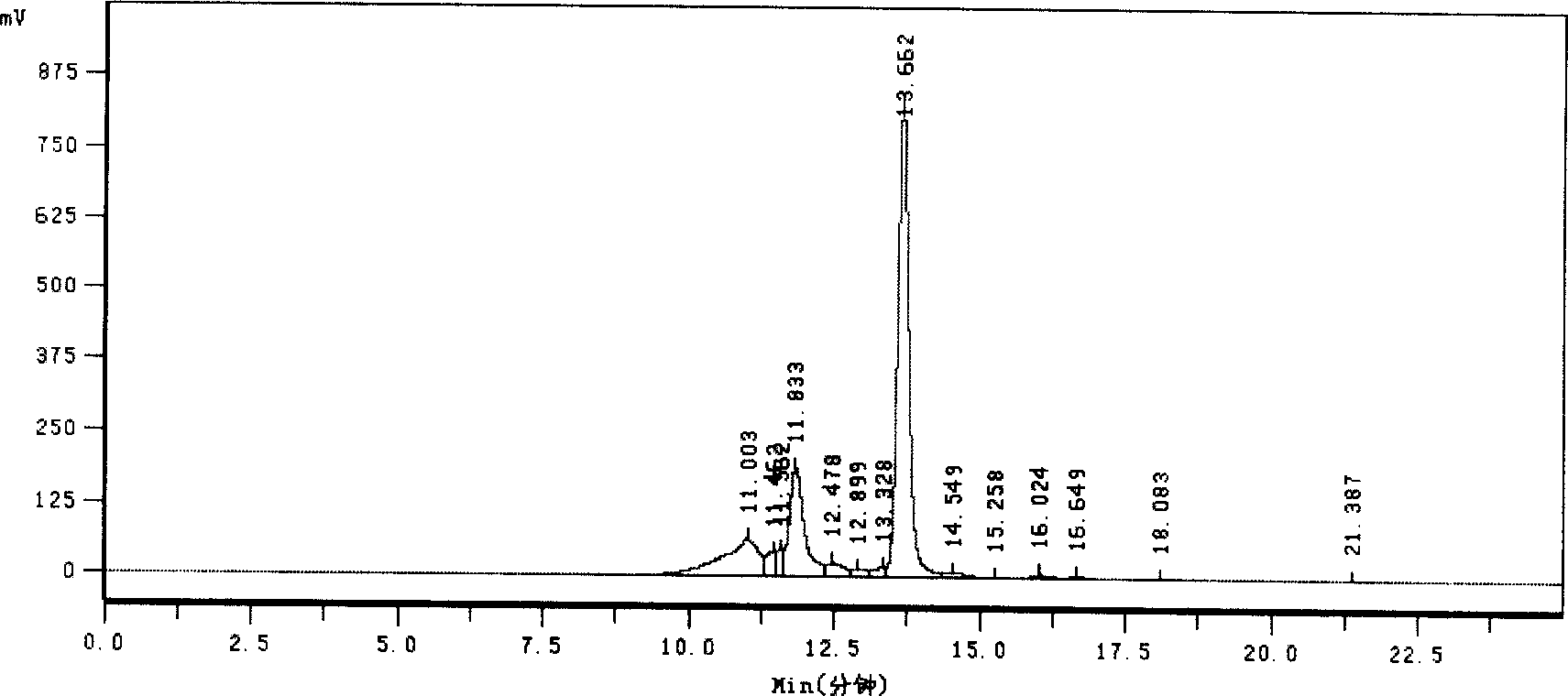
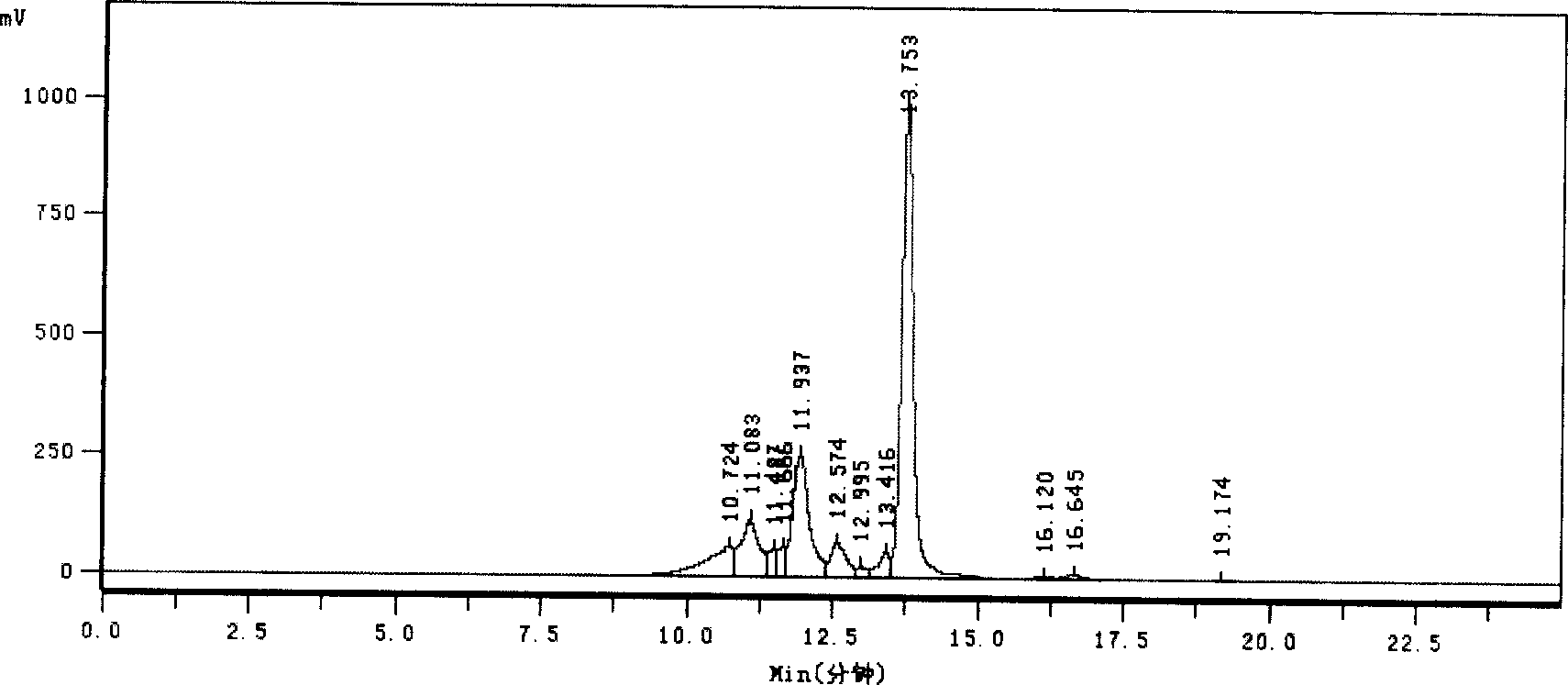
Image
Examples
Embodiment 1
[0029] Embodiment 1, the production of cerebroprotein hydrolyzate (stock solution) for injection
[0030] Take 100.0kg of fresh or frozen healthy pig brains that have passed the inspection, remove non-brain tissues such as connective tissue and bone fragments, wash them with water for injection, drain them, first grind them into pieces with a meat grinder, and adjust the scale to 200 The purpose is to grind the colloid finely, collect the homogenate, add 150.0 kg (1.5 times the weight) of water for injection and stir well. Warm the slurry to 42°C±2°C, adjust the pH to 1.5-1.7 with 4.5% hydrochloric acid, add 10g of pepsin per kg of slurry (1%), add 2.5kg of pepsin, and maintain the pH at 1.5-1.7. Hydrolyze at 42°C±2°C for 8 hours. Use 5M NaOH to adjust the pH of the pepsin hydrolyzed slurry to 7.1-7.3, add 10g of trypsin per kg of the slurry (1%), add 2.5kg of trypsin, stir well, and maintain the pH at 7.5-7.7, 42℃± Hydrolyze at 2°C for 4 hours, heat up to 100°C after enzyma...
Embodiment 2
[0032] Embodiment 2, the production method of cerebroprotein hydrolyzate freeze-dried agent for injection
[0033] (1) Preparation of raw and auxiliary materials
[0034] In a 10,000-class clean area, add the brain protein hydrolyzate stock solution and mannitol that have passed the full inspection into the liquid preparation tank according to the prescription amount, add water for injection to the mark, mix well, add activated carbon according to 0.2% of the liquid volume, and stir Decolorize for 20 minutes, coarsely filter with a 0.45um membrane, and then finely filter with a 0.22um membrane to measure its content, and send it to a 100-grade packing room for use.
[0035] The prescription is: cerebroprotein hydrolyzate stock solution, equivalent to about 30.0g of total nitrogen
[0036] Mannitol 240g
[0037] Add water for injection to 4000.0ml
[0038] Packed into 1000 bottles
[0039] In the prescription, cerebroprotein...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com