Method for preparing 4-formoxylbenzofuran
A technology of dibromomethylbenzene and furazan, which is applied in the new field of preparation of 4-formylbenzofurazan, can solve the problems such as unsuitable for commercial synthesis and laborious
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
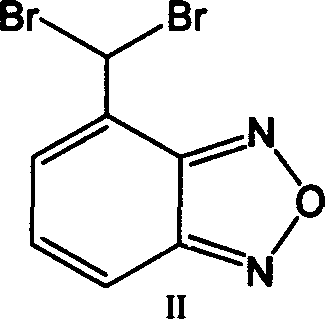
[0027] Embodiment 1: Preparation of 4-dibromomethylbenzofura (II)
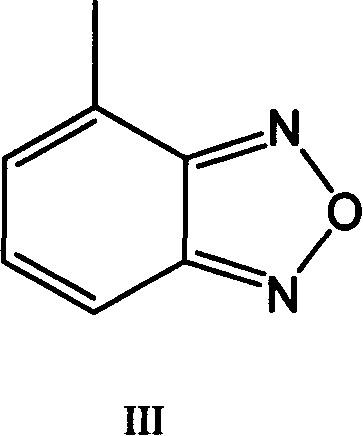
[0028] 4-Methylbenzofura (C 7 h 6 N 2 O) (100.0 g, 0.746 mol) was dissolved in 1.0 liter of chlorobenzene. N-Bromosuccinimide (398.5 g, 2.24 mol), AIBN (36.1 g, 0.149 mol) were added, and the reaction mixture was heated to 80-82 °C over 24 hours. After the reaction was completed, the reaction mixture was cooled to room temperature, filtered, and the filtered solution was washed with water twice. The resulting organic layer was dried over magnesium sulfate and vacuum distilled at 70-80°C. Diisopropyl ether (200 mL) was added to the residue product thus obtained, and stirred at 25°C for 30 minutes. The resulting product was filtered and dried under vacuum at 50-60°C. The resulting 4-(dibromomethyl)benzofura (II) was 160 grams, and by HPLC analysis, the purity varied between 97.0-99.0%, and the 4-(bromomethyl)benzofura(IV) ) content is less than 5%. 4-(Dibromomethyl)benzofura(II) was further characterized...
Embodiment 2
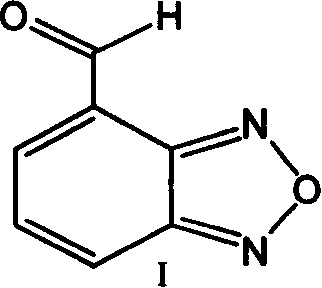
[0029] Embodiment 2: Preparation of 4-formylbenzofura (I)
[0030] 4-(Dibromomethyl)benzofura(II) (160.0 g, 0.342 mol) obtained in Example 1 was suspended in 800 ml of acetic acid and heated to 90°C. 2 L of HCl was slowly added to the above solution over a period of 8 hours while maintaining the temperature. This temperature was further maintained at 90°C. After the reaction was complete, the reaction mixture was cooled to room temperature. The reaction mixture was then cooled to 25°C and diluted with 1.6 liters of water. The product was extracted with 2 x 500 mL of dichloromethane. The organic layer was dried over sodium sulfate and vacuum distilled at 35-40°C to give 41 g of solid product. HPLC purity -99.0%. The product was further characterized using NMR and mass spectrometry techniques. NMR in CDCl 3 Inside, C-10: 1H (formyl) at δ 10.4, singlet; C-4: 1H δ 8.2 doublet; C-6: 1H δ 8.0 doublet; δC-5: 1H δ 7.6 triplet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com