Gefitinib tablet preparation method
A technology of gefitinib and tablets, which is applied in the field of antineoplastic drugs, gefitinib tablets and its preparation, can solve the problems of low drug dissolution rate, gap in release effect of Iressa, and low f2 value, and achieve Good commercial prospects, good stability, high in vitro dissolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1 Gefitinib micronization treatment
[0054] 10 kg of gefitinib raw material was placed in a jet mill (Kunshan Miyou Group Co., Ltd.) for micronization until 8.73 kg of micronized gefitinib had a yield of 87.3%. After micronization, a small sample of gefitinib was taken and detected by a Mastersizer 2000 Malvern laser particle size analyzer (Malvern Instrument Co., Ltd., UK), and the D90 was 8.488 μm.
Embodiment 2
[0055] Example 2 Gefitinib micronization treatment
[0056] 10 kg of gefitinib raw material was placed in a jet mill (Kunshan Miyou Group Co., Ltd.) for micronization until 9.21 kg of micronized gefitinib was obtained, and the yield was 92.1%. Take the micronized gefitinib sample and detect it with a Mastersizer 2000 Malvern laser particle size analyzer (Malvern Instrument Co., Ltd., UK), and the D90 is 9.796 μm.
Embodiment 3
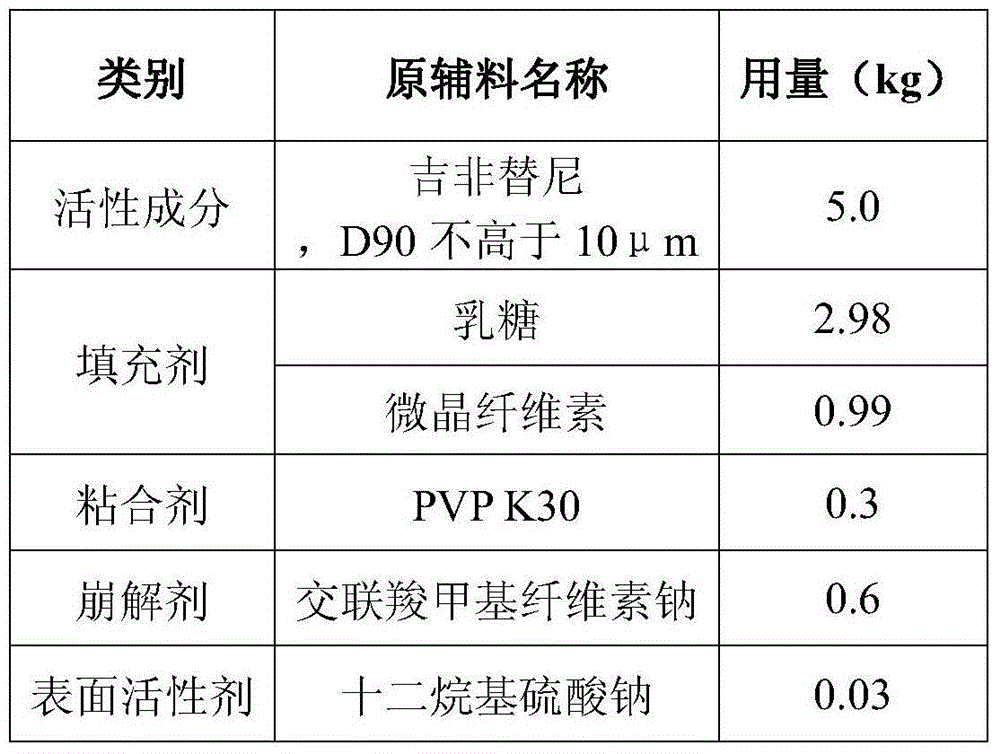
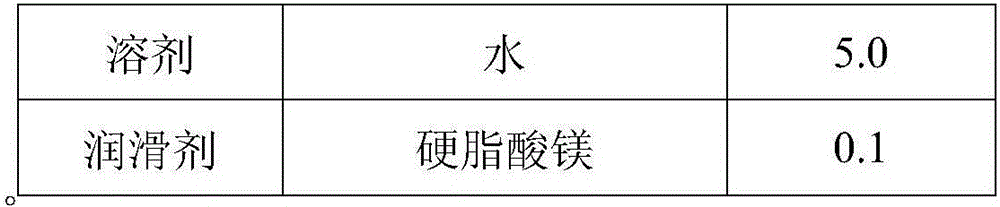
[0057] The preparation of embodiment 3 gefitinib tablets
[0058] A. Preparation of Gefitinib Tablets
[0059] Weigh 5.0kg of purified water, add 0.3kg of povidone K30 under stirring and stir evenly to prepare a clear and transparent solution, add sodium lauryl sulfate while stirring, stir evenly, and set aside; take the raw and auxiliary materials according to the prescription in Table 3, Put the processed raw and auxiliary materials into the G6 experimental multifunctional wet mixing granulator, stir at a low speed for 300 seconds, so that the raw and auxiliary materials are mixed evenly; add the binder within 120 seconds under the low-speed stirring state, and finally shear at a low speed for 20 seconds Seconds to make soft materials with uniform particle size; discharge, put the soft materials in a swing granulator to granulate at 20 mesh; dry the wet granules at 50±5°C until the moisture content is 1% to 3%, and finish at 20 mesh. Pack it in a PE bag, weigh it, add a cer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com