Preparation method of tolvaptan tablet
A protan tablet and tablet compression technology, applied in the field of chemical pharmacy, can solve the problems of poor drug bioavailability and clinical curative effect, low dissolution rate of tolvaptan tablet in vitro, large investment in equipment, etc. Utilization, good clinical efficacy, good effect of bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
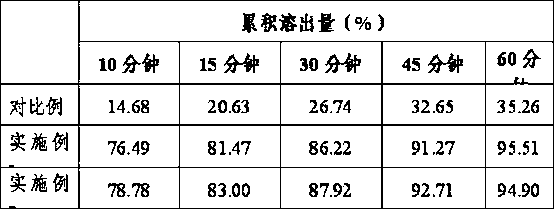
Image
Examples
Embodiment 1
[0019] Embodiment 1: 1, the raw material of following recipe quantity is made tolvaptan spray powder,
[0020] Tolvaptan 50g Highly substituted-hydroxypropyl cellulose 15g Absolute ethanol 195ml Dichloromethane 455ml
[0021] Preparation:
[0022] A. Pass tolvaptan and highly substituted-hydroxypropyl cellulose through an 80-mesh sieve and mix well.
[0023] B. Take absolute ethanol and dichloromethane according to the prescription and mix them evenly.
[0024] C. Dissolving the mixture in step A in the above solvent.
[0025] D. Spray-dry the mixture in step C into powder, the inlet air temperature: 150-160°C, the outlet air temperature: 80-90°C.
[0026] 2, make 200 tolvaptan tablets with the raw and auxiliary materials of following prescription quantity,
[0027] prescription Dosage (g) Tolvaptan Spray Powder 3.9 lactose 7.4 microcrystalline cellulose 4.14 Hydroxypropylmethylcellulose 0.6 Cross-li...
Embodiment 2
[0030] Embodiment 2: 1, the preparation of tolvaptan spray powder is the same as embodiment 1.
[0031] 2, make 200 tolvaptan tablets with the raw and auxiliary materials of following prescription quantity,
[0032] prescription Dosage Tolvaptan Spray Powder 3.9 lactose 7.4 microcrystalline cellulose 3.54 Hydroxypropylmethylcellulose 0.6 Cross-linked polyvinylpyrrolidone 1.5 water Appropriate amount Magnesium stearate 0.16
[0033] Preparation method: pass the tolvaptan spray powder through a 200-mesh sieve, pass the auxiliary materials through a 100-mesh sieve, and set aside. Accurately weigh the raw materials and all internal auxiliary materials according to the prescription amount, mix them evenly, add appropriate amount of water to prepare soft materials, and pass through a 20-mesh sieve to prepare wet granules; dry at 60°C, pass through a 18-mesh sieve for granulation, and add the prescribed amount of stearin Mag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com