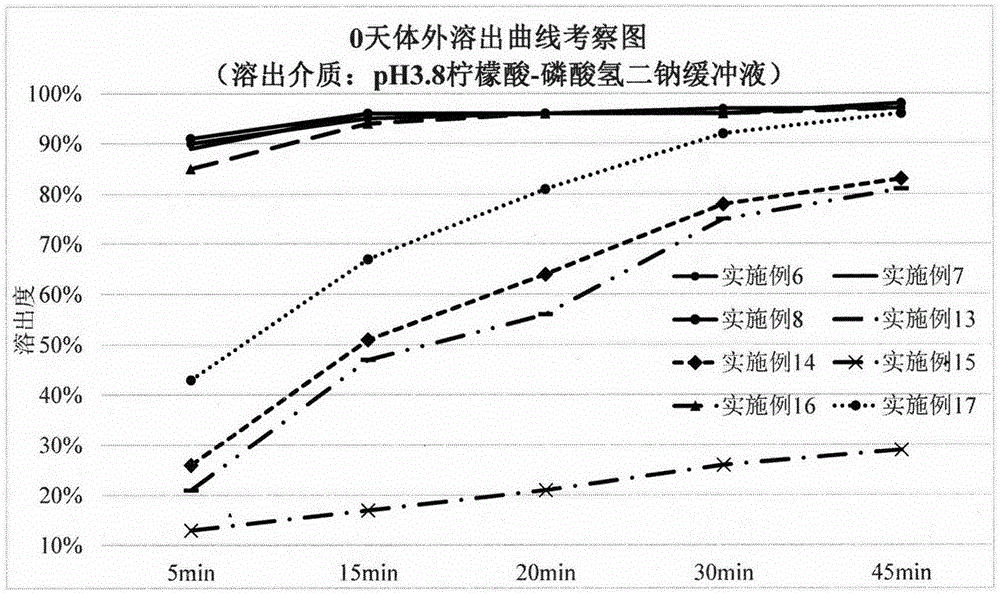
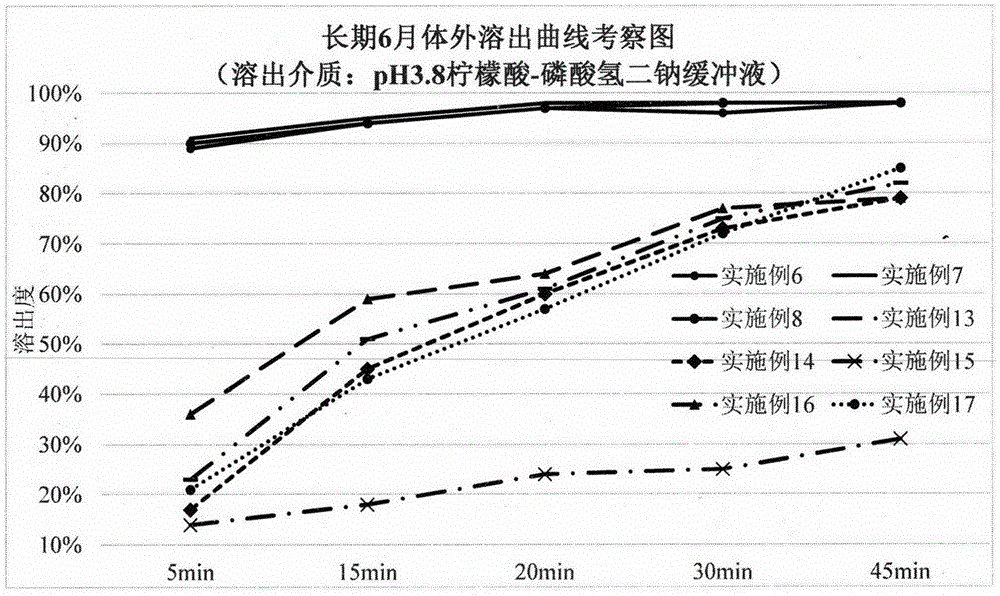
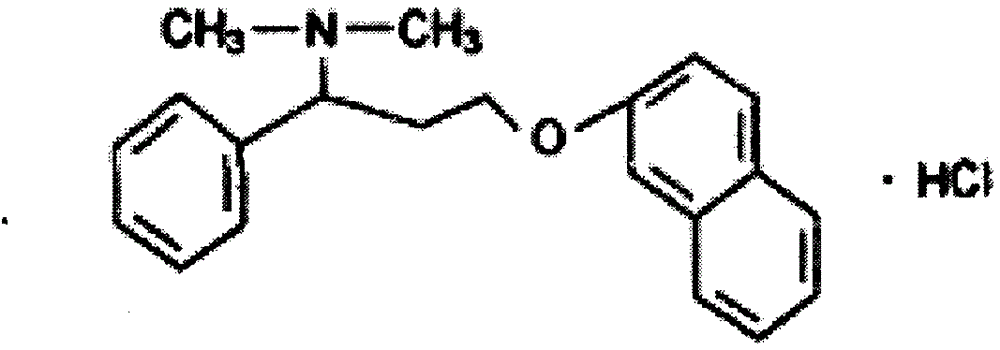
Hydrochloric acid dapoxetine tablet
A technology for dapoxetine hydrochloride and cetine tablets, which is applied in the fields of pill delivery, organic active ingredients, non-effective ingredients of polymer compounds, etc., and can solve the problem of high manufacturing cost and use cost, crystal form A and crystal form B Poor stability, poor stability of amorphous substances, etc., to achieve the effect of stable in vitro dissolution curve, small environmental impact, and no decrease in dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] prescription:
[0023]
[0024]
[0025] Preparation method: Mix dapoxetine hydrochloride and ethanol to form a clear solution, then add it to dextrin to granulate, dry, mix with mannitol, micro-powder silica gel, and magnesium stearate, and then directly compress into tablets.
Embodiment 2
[0027] prescription:
[0028] prescription:
[0029]
[0030] Preparation method: Mix dapoxetine hydrochloride and ethanol to form a clear solution, then add it to dextrin to granulate, dry, mix with mannitol, micro-powder silica gel, and magnesium stearate, and then directly compress into tablets.
Embodiment 3
[0032] prescription:
[0033]
[0034] Preparation method: Mix dapoxetine hydrochloride and ethanol to form a clear solution, then add it to dextrin to granulate, dry, mix with mannitol, micro-powder silica gel, and magnesium stearate, and then directly compress into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com