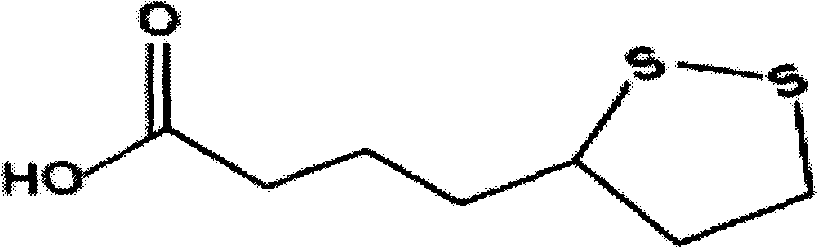
Lipoic acid capsules as well as preparation process and application thereof
A technology of lipoic acid and capsules, which is applied in the direction of capsule delivery, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of poor oral absorption of lipoic acid, low bioavailability, and large side effects, etc. Achieve the effects of improving in vitro dissolution rate, reducing the dosage of active ingredients and maintaining drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
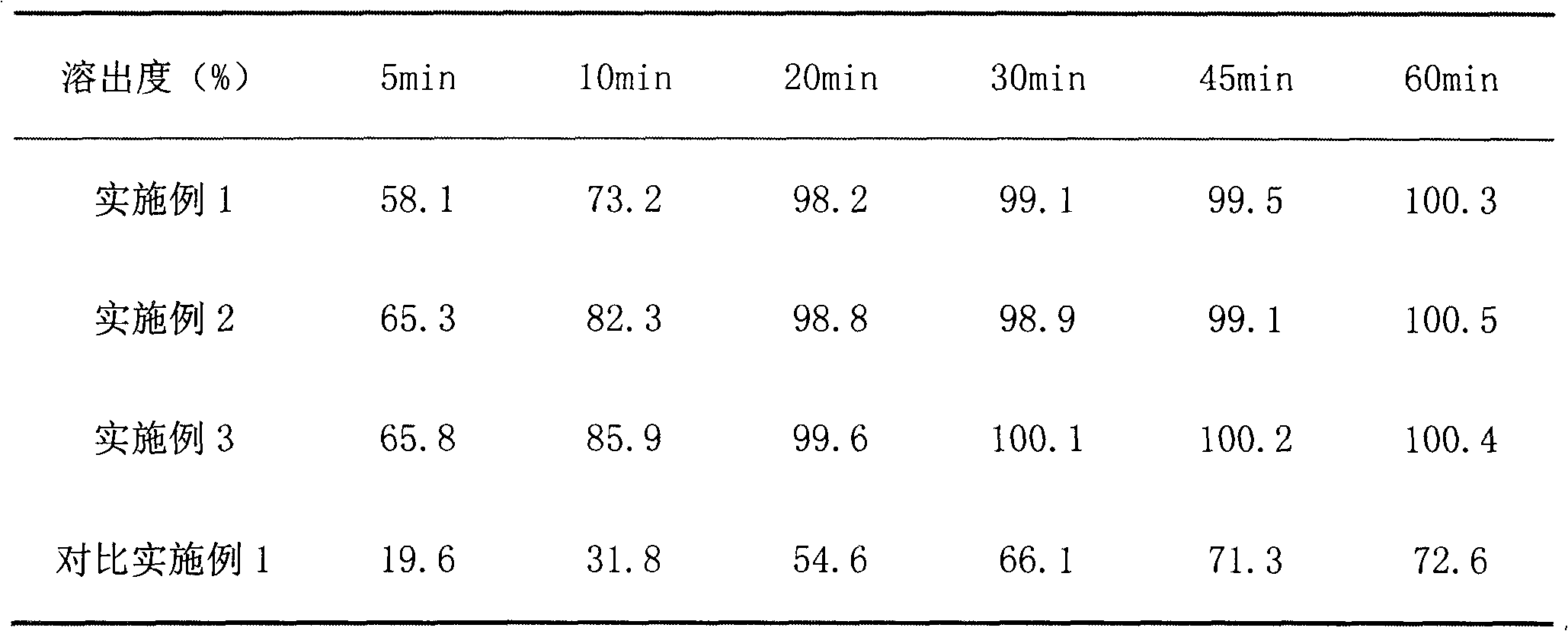
Examples
Embodiment 1
[0019] The preparation of embodiment 1 lipoic acid capsule
[0020] 1. Take 100 g of lipoic acid, micronize it, and pass it through a 200-mesh sieve to get lipoic acid fine powder.
[0021] 2. Put 50g of Poloxamer 237 in a suitable container, put it in a water bath at 49°C-50°C and heat it to a molten liquid state, add 100g of lipoic acid fine powder, and stir rapidly and fully at 49°C-50°C until it is evenly mixed. Let stand to remove air bubbles, spread the mixture into a thin layer and put it in a -5°C refrigerator for rapid cooling. After the mixture is completely solidified, take it out and pulverize, and place it in a vacuum dryer for drying at 35°C for 24 hours, and pulverize it through a 100-mesh sieve to obtain lipoic acid. Solid dispersion.
[0022] 3. Add microcrystalline cellulose 82g, lactose 10g, sodium carboxymethyl starch 8g, and magnesium stearate 4g to the lipoic acid solid dispersion in step 2, and the above-mentioned fillers, disintegrating agents, and lub...
Embodiment 2
[0023] The preparation of embodiment 2 lipoic acid capsules
[0024] 1. Take 100 g of lipoic acid, micronize it, and pass it through a 200-mesh sieve to get lipoic acid fine powder.
[0025] 2. Take 100g of Poloxamer 237 and put it in a suitable container, put it in a water bath at 49°C-50°C and heat it to a molten liquid state, add 100g of lipoic acid fine powder, and stir rapidly and fully at 49°C-50°C until the mixture is uniform. Let stand to remove air bubbles, spread the mixture into a thin layer and put it in a -5°C refrigerator for rapid cooling. After the mixture is completely solidified, take it out and pulverize, and place it in a vacuum dryer for drying at 35°C for 24 hours, and pulverize it through a 100-mesh sieve to obtain lipoic acid. Solid dispersion.
[0026] 3. Add microcrystalline cellulose 82g, lactose 10g, sodium carboxymethyl starch 8g, and magnesium stearate 4g to the lipoic acid solid dispersion in step 2, and the above-mentioned fillers, disintegrati...
Embodiment 3
[0027] The preparation of embodiment 3 lipoic acid capsules
[0028] 1. Take 100 g of lipoic acid, micronize it, and pass it through a 200-mesh sieve to get lipoic acid fine powder.
[0029] 2. Take 33.3g of Poloxamer 237 and put it in a suitable container, put it in a water bath at 49°C-50°C and heat it to a molten liquid state, add 100g of lipoic acid fine powder, and stir quickly and fully at 49°C-50°C until it is evenly mixed. , let stand to remove air bubbles, spread the mixture into a thin layer and put it in a -5℃ refrigerator for rapid cooling, after the mixture is completely solidified, take it out and pulverize, and place it in a vacuum dryer for drying at 35℃ for 24h, and pulverize it through a 100-mesh sieve to obtain sulfur Caprylic acid solid dispersion.
[0030] 3. Add microcrystalline cellulose 82g, lactose 10g, sodium carboxymethyl starch 8g, and magnesium stearate 4g to the lipoic acid solid dispersion in step 2, and the above-mentioned fillers, disintegrati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com