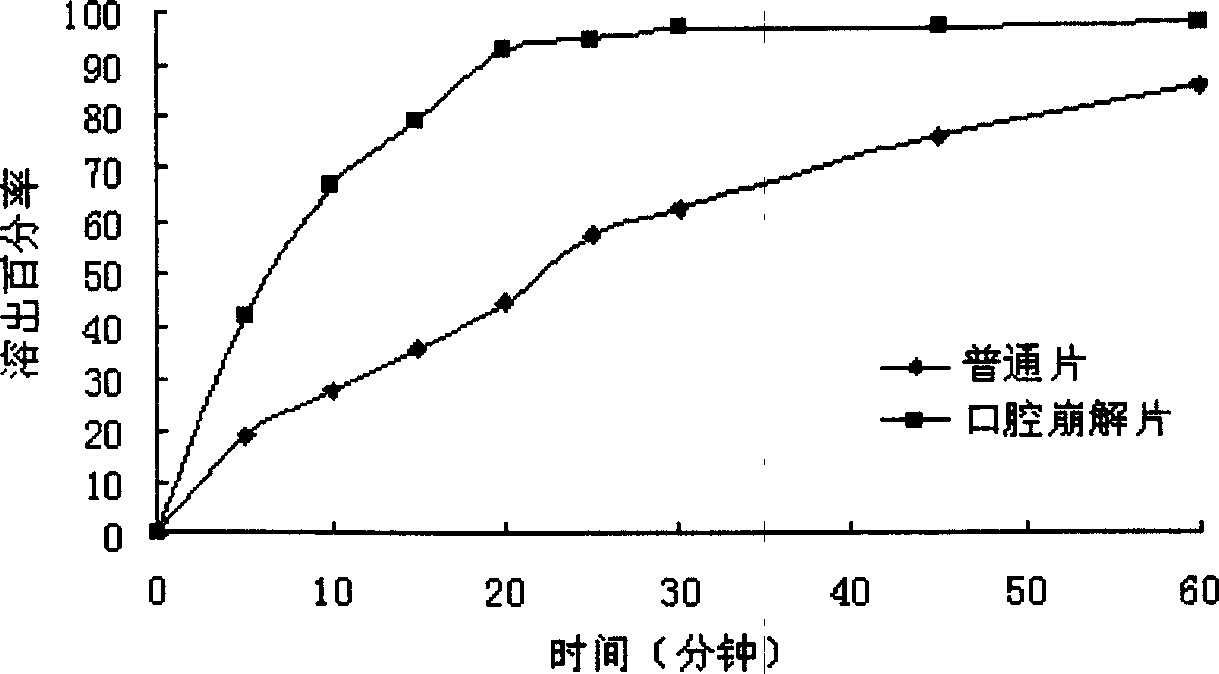
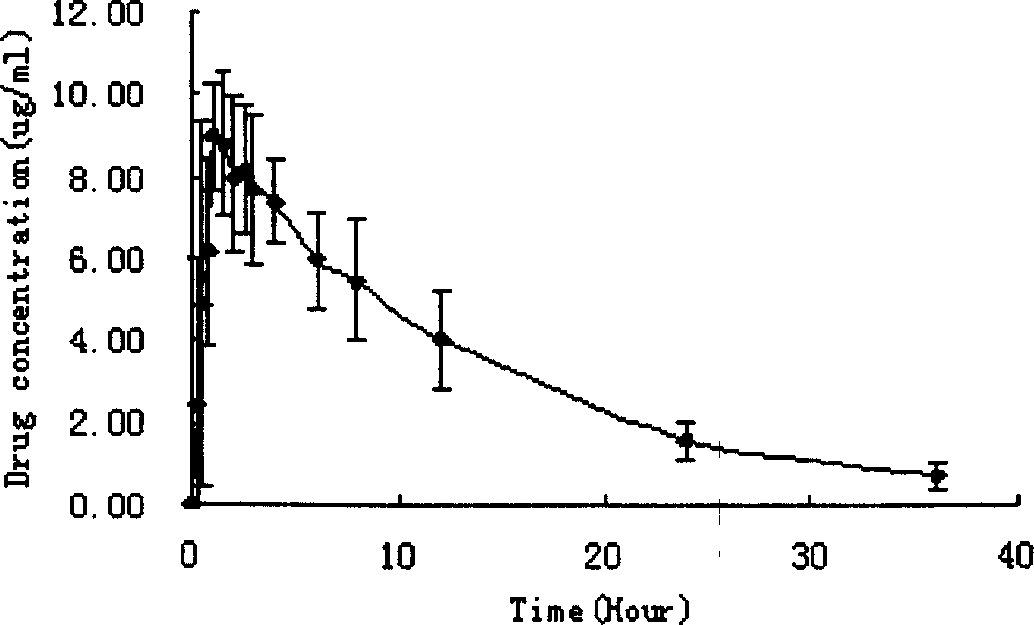
Oral gatifloxacin disintegrant and its preparing process
A technology for oral disintegrating tablets and gatifloxacin, which is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, and pill delivery, etc., to achieve the effects of good taste, rapid disintegration, and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 composition weight percent content
[0031] Gatifloxacin Hydrochloride 20%
[0032] Acrylic resin E 100 2%
[0033] Acetone 0.5%
[0034] Industrial ethanol 0.5%
[0035] Lactose 6%
[0036] Citric acid 2%
[0037] Sodium Hydroxymethyl Cellulose 20%
[0038] Microcrystalline Cellulose 37%
[0039] Low Substituted Hydroxypropyl Cellulose 7%
[0040] 10% aqueous solution of polyvinylpyrrolidone 3% (calculated as polyvinylpyrrolidone)
[0041] Talc 2%
[0042] Preparation method: powder-coated tablet method: firstly, the above-mentioned excipients are respectively crushed through a 150-mesh sieve, and gatifloxacin hydrochloride is passed through a 200-mesh sieve. Weigh 4g of gatifloxacin hydrochloride fine powder, put it in a fluidized bed to make it boil, coat with a 10% solution of 100mg of acrylic resin E 100 and 100ml of acetone...
Embodiment 2
[0043] Embodiment 2 composition weight percentage content
[0044] Gatifloxacin Hydrochloride 40%
[0045] Hydroxypropyl methylcellulose 7% aqueous solution 7% (calculated as hydroxypropyl methylcellulose)
[0046] Lactose 5.5%
[0047] Aspartame 0.5%
[0048] Sodium Hydroxymethyl Cellulose 10%
[0049] Microcrystalline Cellulose 25%
[0050] Low Substituted Hydroxypropyl Cellulose 7%
[0051] Polyvinylpyrrolidone 10% aqueous solution 4% (calculated as polyvinylpyrrolidone)
[0052] Talc 1%
[0053] Preparation method: powder-coated tablet method: firstly, the above-mentioned excipients are respectively crushed through a 150-mesh sieve, and gatifloxacin hydrochloride is passed through a 200-mesh sieve. Weigh 12g of gatifloxacin hydrochloride fine powder, put it in a fluidized bed to make it boil, dissolve 2.1g of hydroxypropyl methylcellulose into a 7% aqueous solution as ...
Embodiment 3
[0054] Embodiment 3 composition weight percent content
[0055] Gatifloxacin Hydrochloride 50%
[0056] Glyceryl Behenate 5%
[0057] Sorbitol 15%
[0058] Lactose 15%
[0059] Sodium Starch Carboxymethyl 12%
[0060] Vanillin 1%
[0062] Preparation method: powder-coated tablet method: crush gatifloxacin hydrochloride through a 200-mesh sieve, weigh 17.5g of fine powder, put it in a fluidized bed to make it boil, and coat it with 1.75g of behenic acid glyceride. 10% weight gain of the main ingredient, coating the drug through a 120-mesh sieve, weighing 5.25g of sorbitol, 5.25g of lactose, 4.2g of sodium carboxymethyl starch and mixing with the coated drug. Granulate with an 80-mesh sieve, dry, mix the dry granules with 350 mg of vanillin and 700 mg of magnesium stearate, control the tablet weight to 350 mg, and press into tablets to obtain 100 gatifloxacin orally disintegrating tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com