Preparation process of rabeprazole sodium enteric capsules
The technology of rabeprazole sodium and preparation process is applied in the field of pharmacy and can solve the problems of low production efficiency, long production cycle of rabeprazole sodium enteric-coated capsules, and high production cost, so as to improve production efficiency and improve bioavailability , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] The processing of embodiment 1 calcium hydroxide micronization
[0092] Take by weighing the calcium hydrochloride coarse powder 5.06Kg of 80 mesh sieves, put jet mill (the model is QYF-100, manufactured by Miyou Group Co., Ltd.) to micronize, and pass through 200 mesh sieves to obtain micronized hydrochloride Calcium 3.58Kg, yield is 70.8%. Get the calcium hydroxide small sample after micronization, detect with Malvern laser particle size analyzer (model is Mastersizer2000, British Malvern instrument company manufactures), and D90 is 59.6um.
Embodiment 2
[0093] The preparation of embodiment 2 rabeprazole sodium enteric-coated capsules
[0094] A. Preparation of loaded pills
[0095] Weigh sodium hydroxide, rabeprazole sodium, highly substituted hydroxypropyl cellulose L, and Tween 80 successively according to the prescription design amount, dissolve them in 75% ethanol aqueous solution one by one, stir well, and be prepared into a viscose containing active pharmaceutical ingredients. Mixture, standby; Mannitol, low-substituted hydroxypropyl cellulose, calcium hydroxide are crossed 80 mesh sieves respectively, put wet granulator (G6 experimental multifunctional wet mixing granulator, Shenzhen Xinyite Machinery Co., Ltd. company), set the speed at 3 rpm, and mix for 5 minutes; add an appropriate amount of adhesive to the mixed material within 3 minutes, stir for 1 to 3 minutes, and shear at a speed of 5 to 10 rpm. Cut for 1 to 2 minutes to make a soft material; put the prepared soft material into an extruder (CGC-350 multifunct...
Embodiment 3
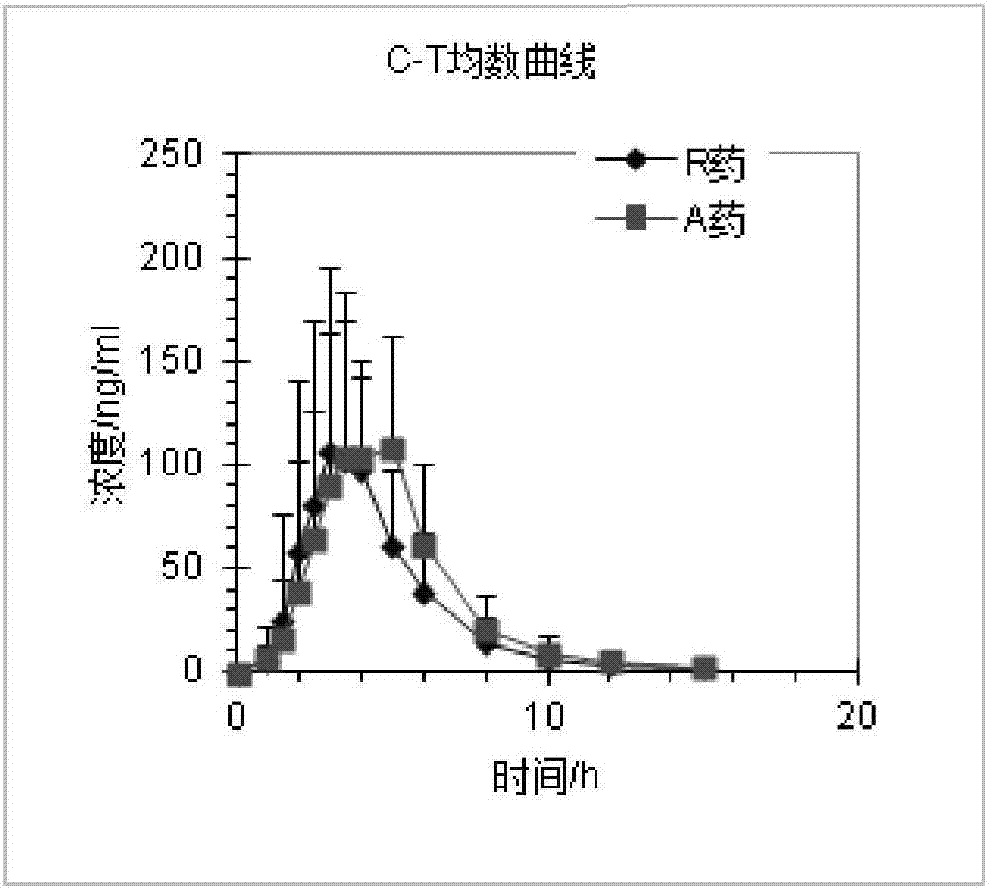
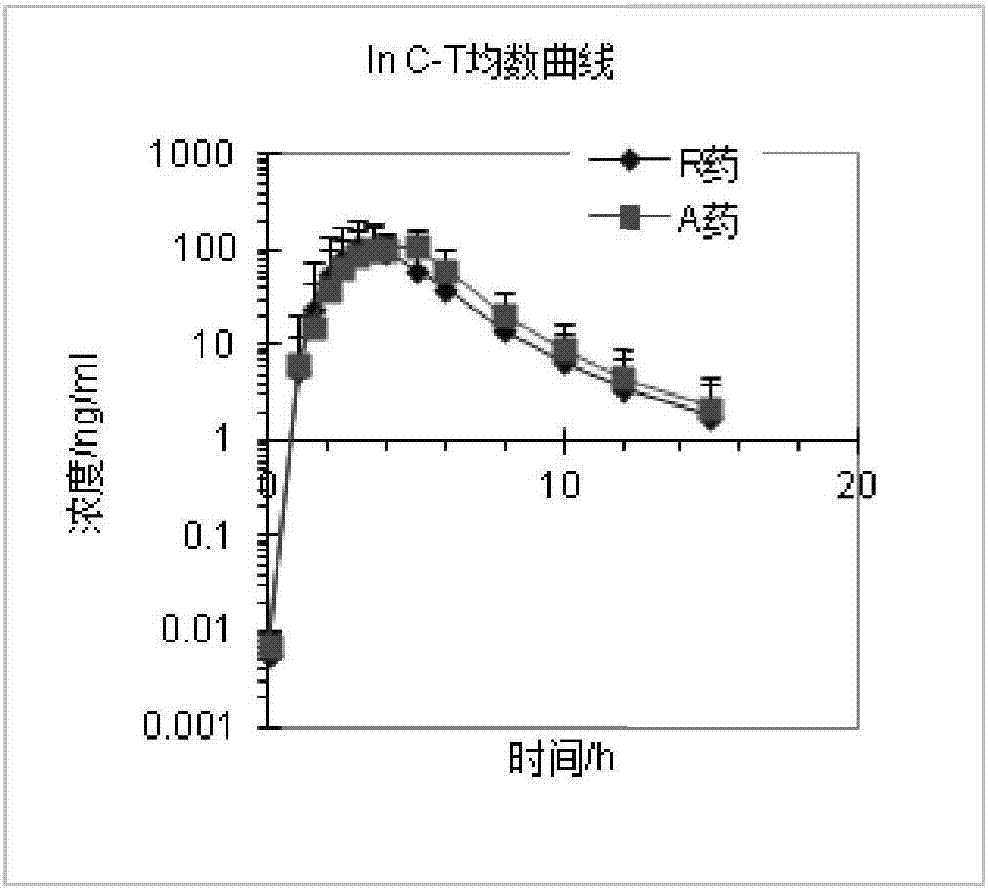
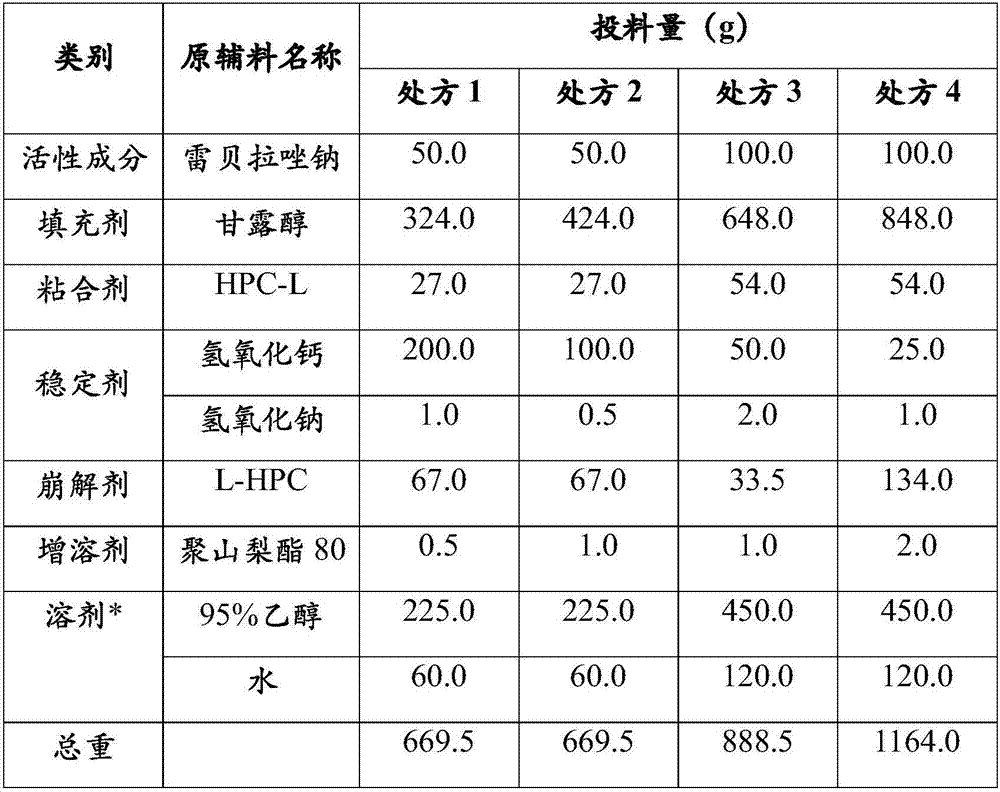
[0113] Example 3 Rabeprazole Sodium Enteric-Coated Capsules (Prescription 1, Prescription 2, Prescription 3, Prescription 4) prepared by the present invention are compared with the in vitro dissolution rate of the reference preparation (R)
[0114] Reference preparation (R): rabeprazole sodium enteric-coated capsules (trade name: ACIPHEX SPRINKLE).
[0115] Get each 6 rabeprazole enteric-coated capsules (prescription 1, prescription 2, prescription 3, prescription 4) and reference preparation (R) prepared in Example 2, detect its pH6.8 phosphate buffer saline by the following method in vitro dissolution rate.
[0116] Dissolution method: paddle method
[0117] (1) Acid medium: 0.1M hydrochloric acid solution
[0118] Acid medium volume: 750ml
[0119] Number of rotations: 100 rpm
[0120] Stop time: 120min
[0121] (2) Dissolution medium: pH6.8 buffer
[0122] Medium volume: 1000ml
[0123] Number of rotations: 50 rpm
[0124] Sampling time: 5, 10, 15, 20, 30, 45, 60 m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Medium volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com