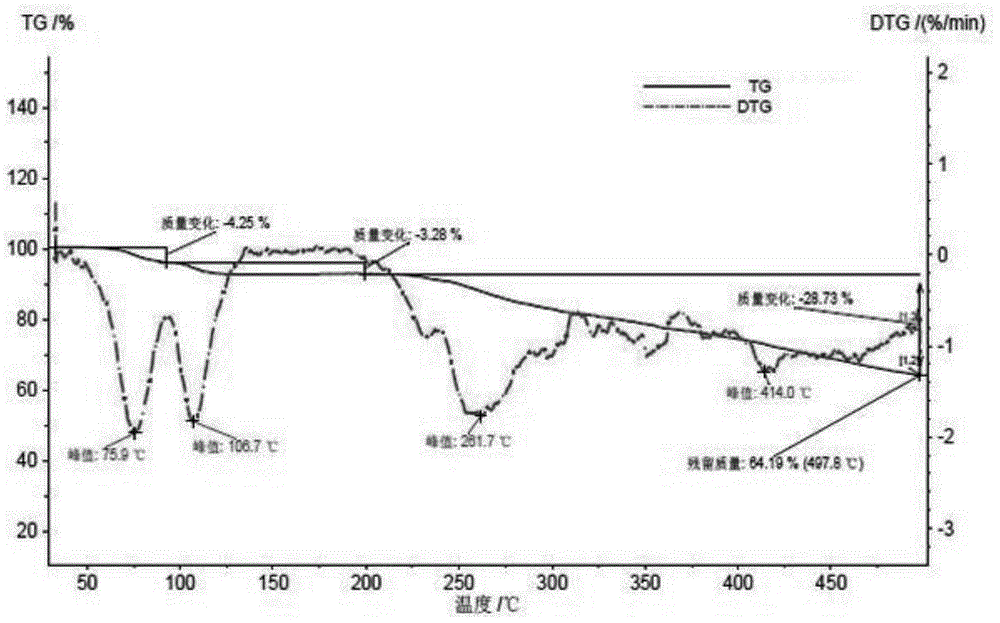
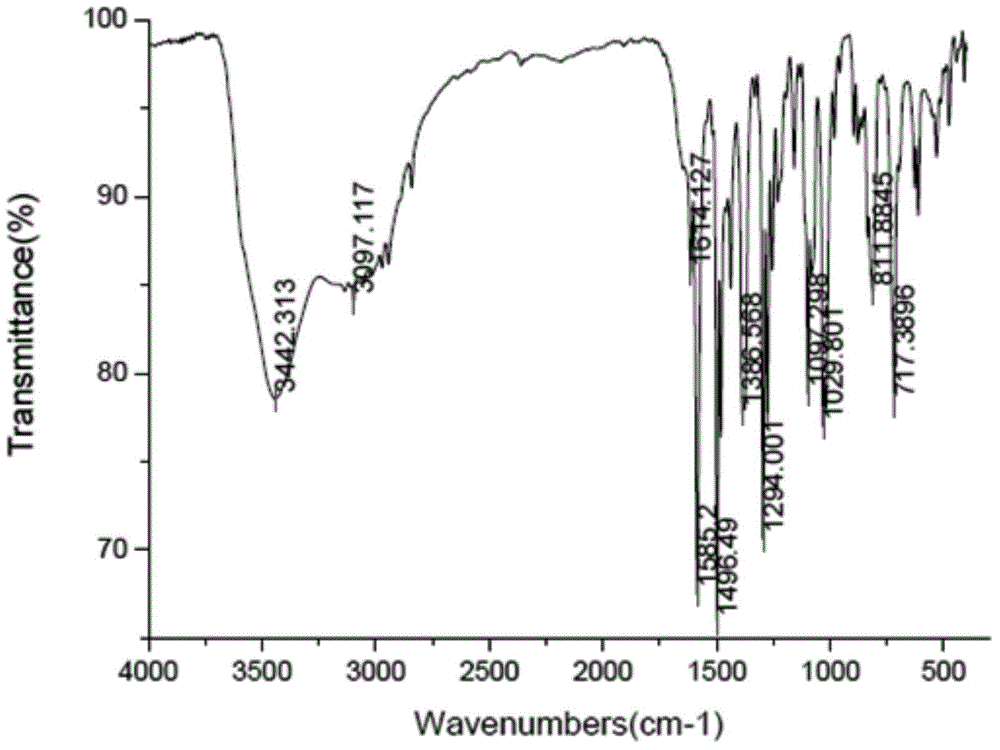
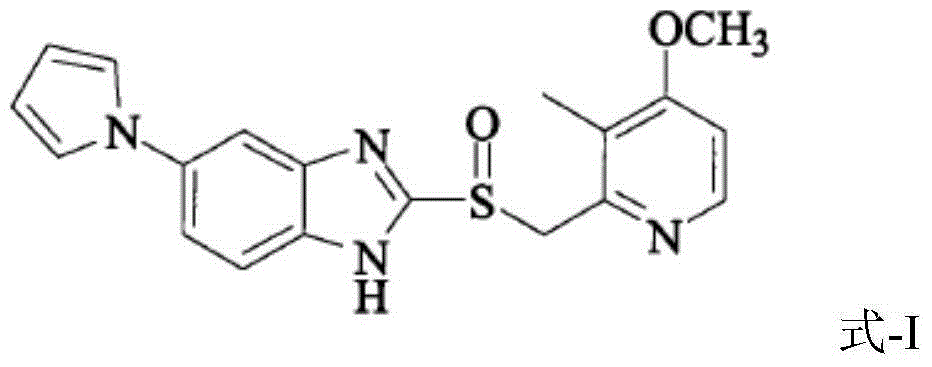
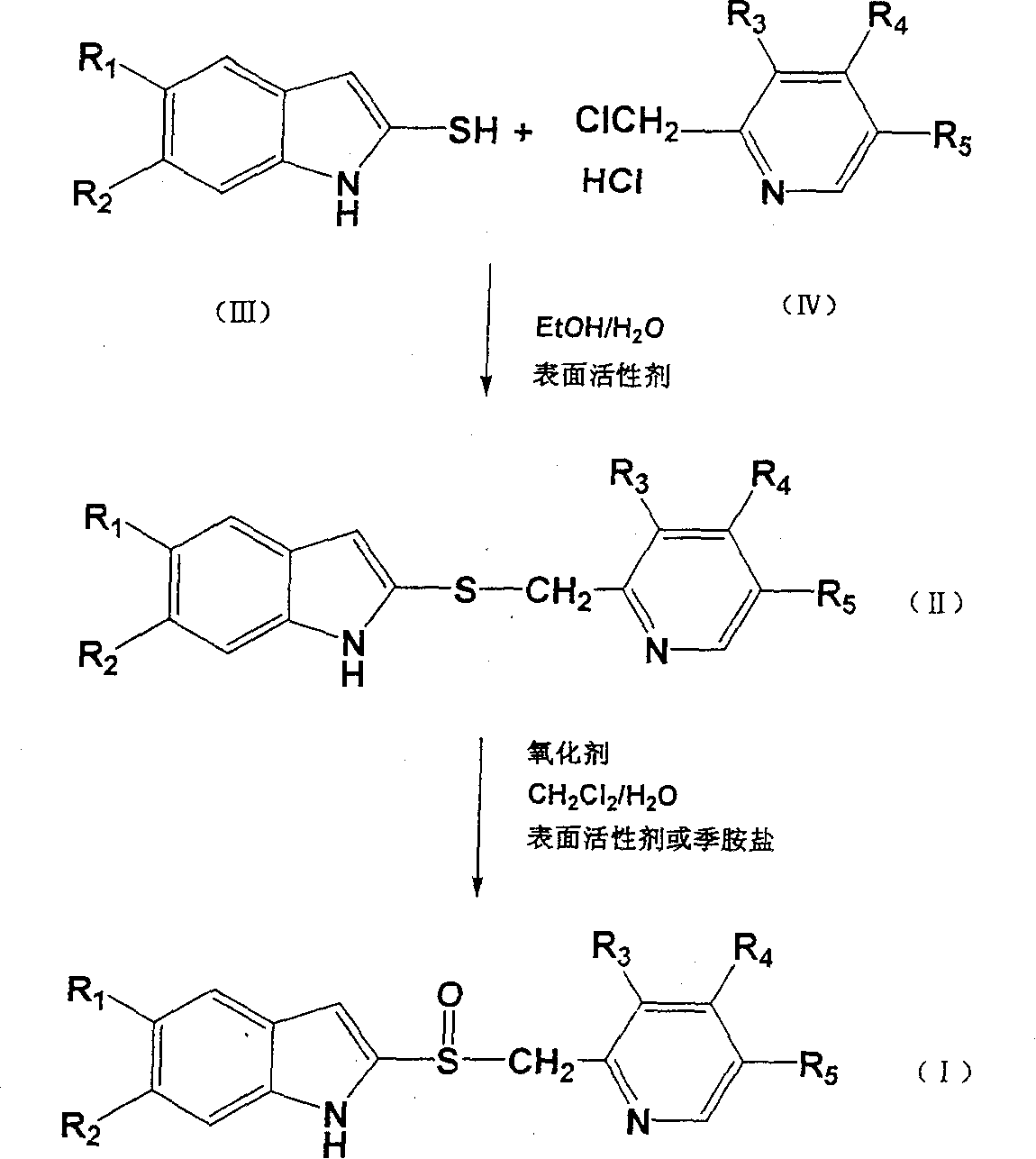
Patents
Literature
92 results about "Ilaprazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
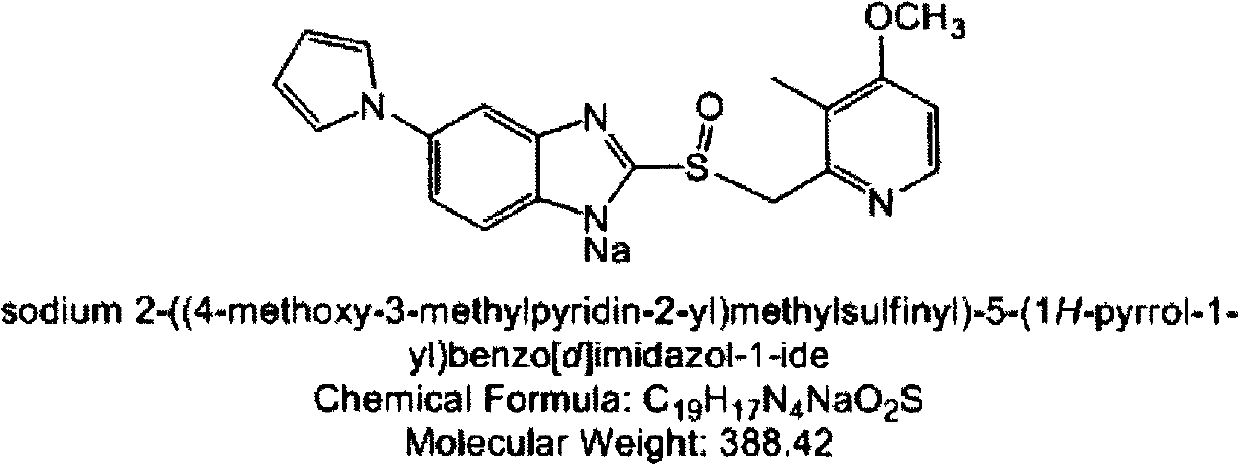
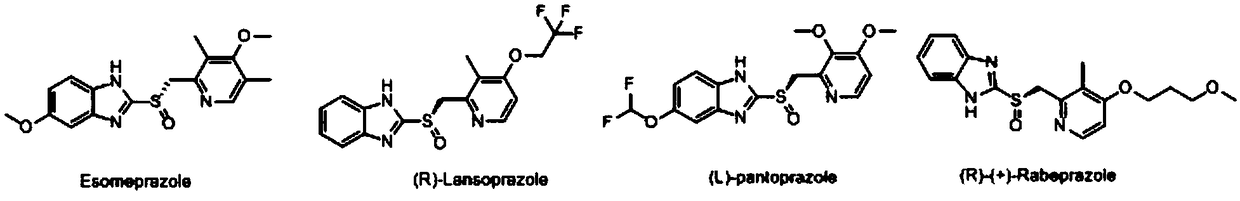
Ilaprazole (trade name Noltec) is a proton pump inhibitor (PPI) used in the treatment of dyspepsia, peptic ulcer disease (PUD), gastroesophageal reflux disease (GORD/GERD) and duodenal ulcer. It is available in strengths of 5, 10, and 20 mg.
Powder injection for treating peptic ulcers and preparation method thereof
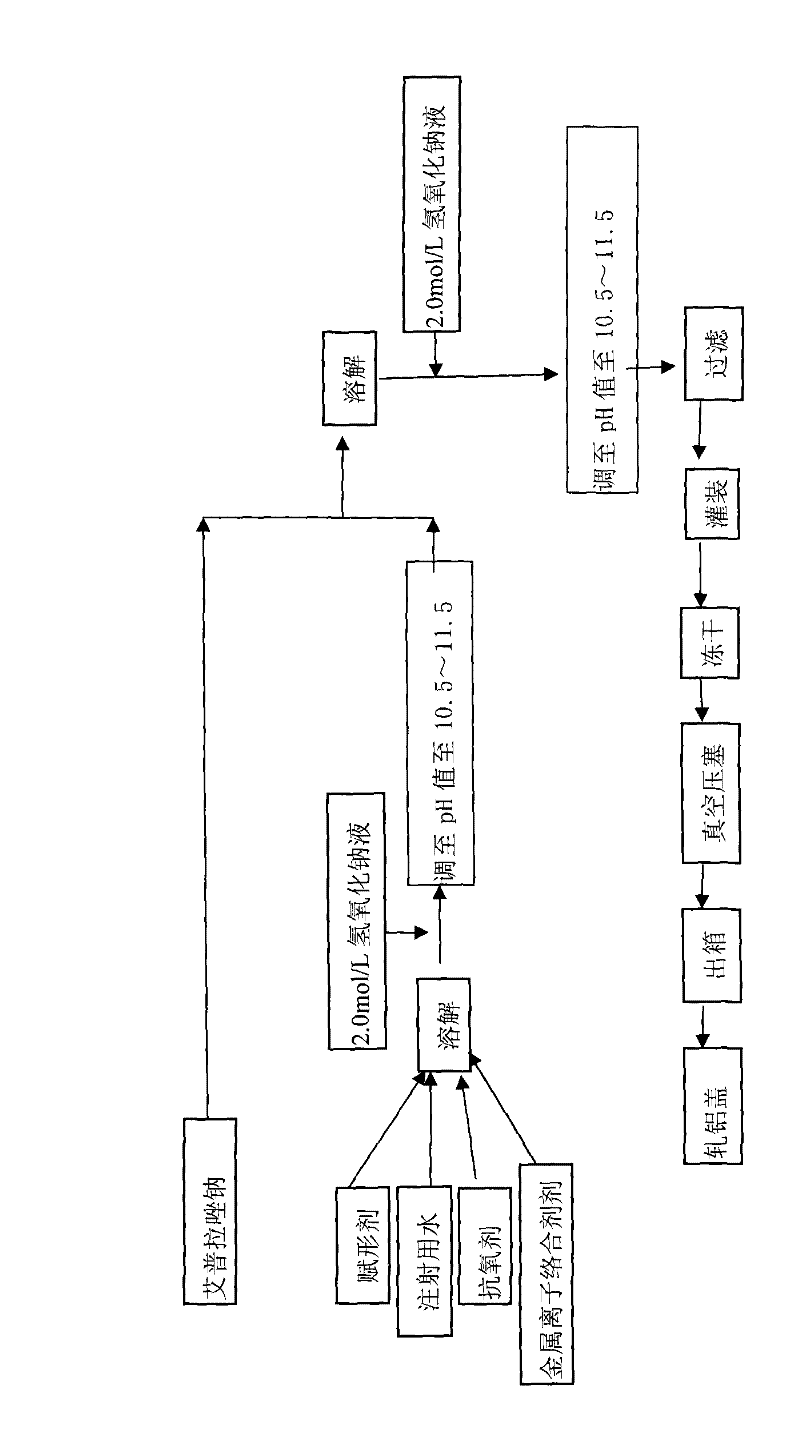
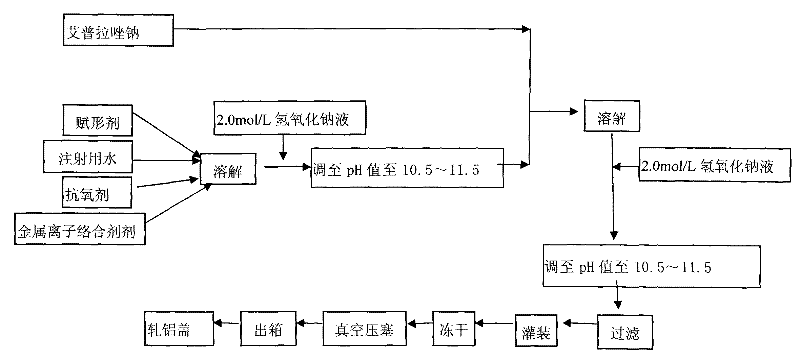
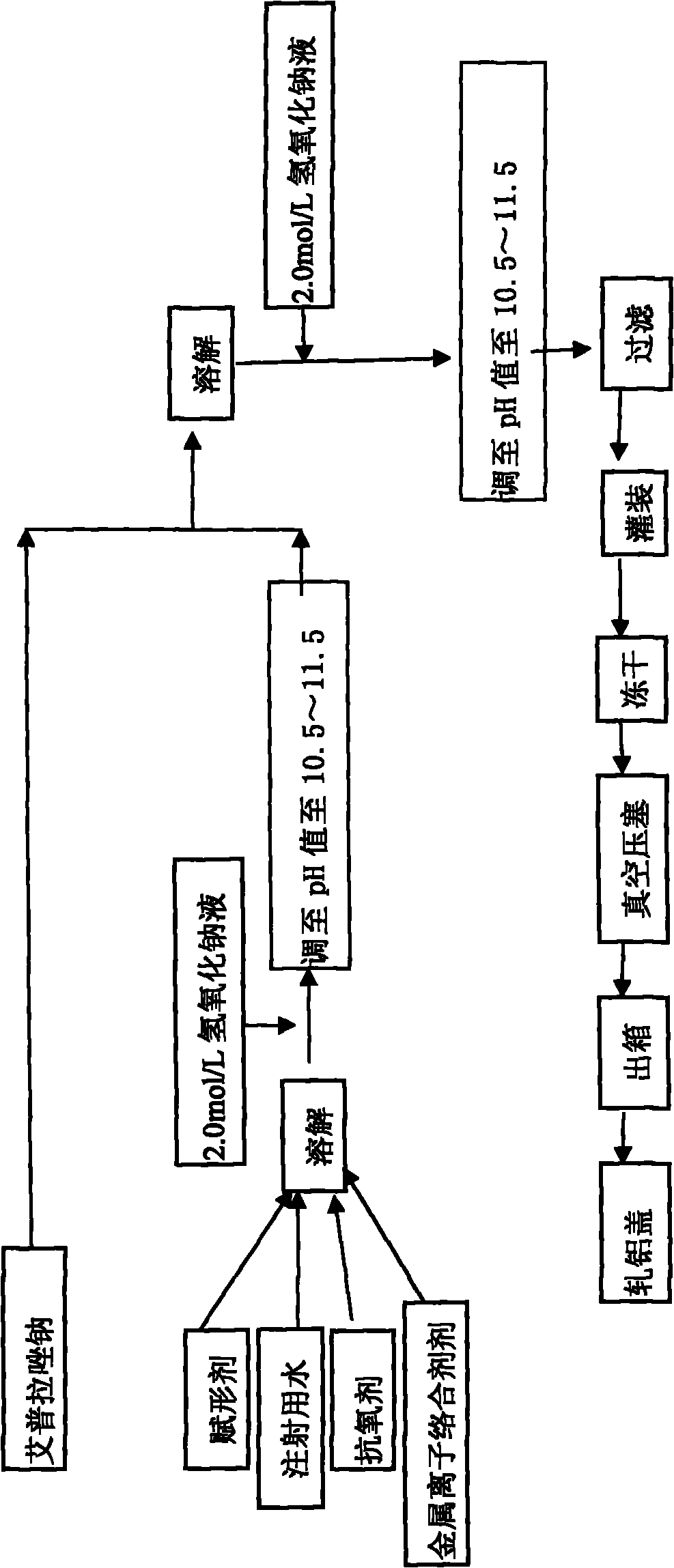
The invention provides an injection for treating a peptic ulcers and a preparation method thereof. The powder injection provided by the invention comprises the active ingredients of Ilaprazole sodium and excipient, wherein the ratio of the both in parts by weight is (1:1)-(1:30), and the preferable ratio is (1:10)-(1:18). The preferable prescription of the powder injection provided by the invention comprises 1 part of Ilaprazole sodium, 1-30 parts of excipient, 0-10 parts of antioxidant and / or 0-0.3 part of metal ion complexing agent; and a right amount of inorganic base is added to regulate the pH value to 9.0-12.0. The Ilaprazole sodium freeze-dried powder injection provided by the invention has stable quality, and is suitable for treating peptic ulcer bleeding and stress ulcers and preventing upper gastrointestinal bleeding caused by serious diseases.
Owner:LIVZON PHARM GRP INC
Ilaprazole enteric-coated tablets and preparation method thereof
InactiveCN102525990AImprove acid resistanceWidely distributedOrganic active ingredientsDigestive systemActive agentSurface-active agents
The invention provides ilaprazole enteric-coated tablets and a preparation method thereof. Each enteric-coated tablet contains an enteric-coated pellet and pharmaceutically acceptable tablet excipients, wherein the enteric-coated pellet contains a pellet layer, a medicine loading layer, an isolation layer and an enteric coating layer; and the medicine loading layer contains ilaprazole or pharmaceutically acceptable salt thereof and a stabilizer. The enteric-coated pellet tablets prepared from the ilaprazole have good acid resistance; barrier substances such as an antiacid, a surfactant, an organic solvent, a hydrophobic substance and the like are not contained in the prescription, so that the enteric-coated pellet tablets have health benefits and are safe; the preparation method is easy to operate, the organic solvent is not used, and an active substance is quickly and stably released; and in addition, the pellets in the tablets can be widely and uniformly distributed in an intestinal tract after being taken, the dose dumping is dispersed, and the distribution area of a medicine on the surface of the intestinal tract is increased, so the irritation of the medicine to the intestinal tract can be reduced or eliminated, and the bioavailability of the medicine can be improved.
Owner:LIVZON PHARM GRP INC
Powder injection for treating peptic ulcers and preparation method thereof
The invention provides an injection for treating a peptic ulcers and a preparation method thereof. The powder injection provided by the invention comprises the active ingredients of Ilaprazole sodium and excipient, wherein the ratio of the both in parts by weight is (1:1)-(1:30), and the preferable ratio is (1:10)-(1:18). The preferable prescription of the powder injection provided by the invention comprises 1 part of Ilaprazole sodium, 1-30 parts of excipient, 0-10 parts of antioxidant and / or 0-0.3 part of metal ion complexing agent; and a right amount of inorganic base is added to regulate the pH value to 9.0-12.0. The Ilaprazole sodium freeze-dried powder injection provided by the invention has stable quality, and is suitable for treating peptic ulcer bleeding and stress ulcers and preventing upper gastrointestinal bleeding caused by serious diseases.
Owner:LIVZON PHARM GRP INC
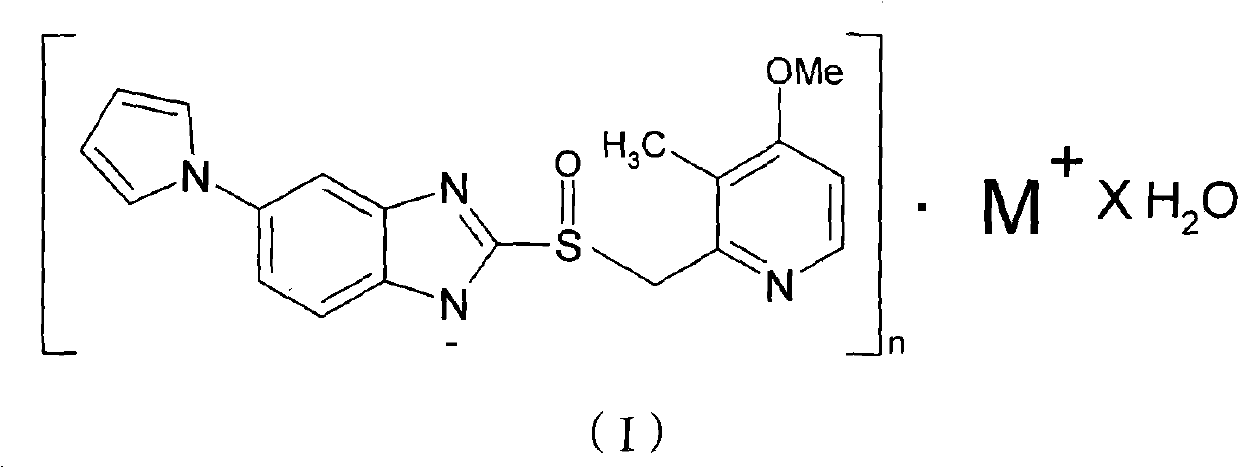
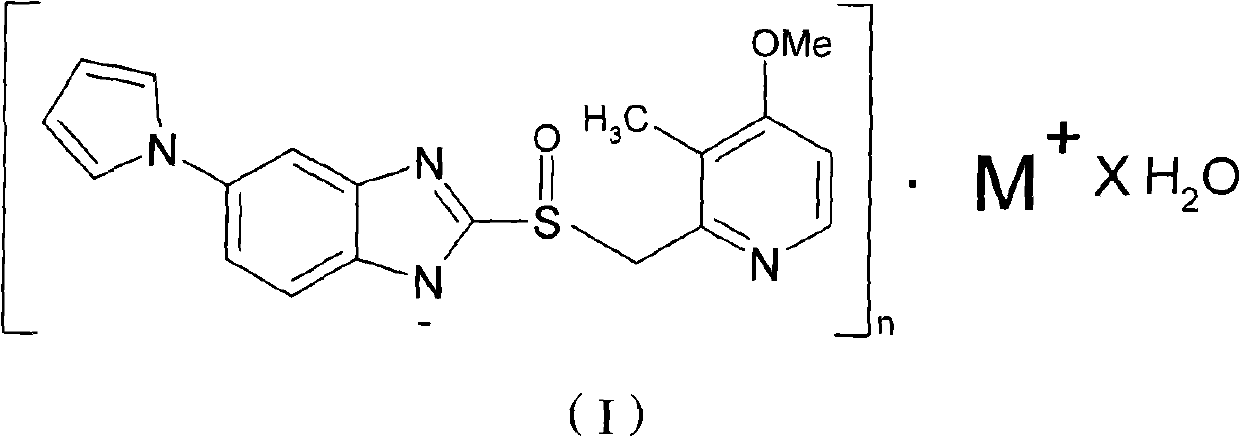
Hydrate of ilaprazole salt, preparation method thereof and application thereof
ActiveCN102140092AGood treatment effectLittle side effectsOrganic active ingredientsOrganic chemistrySide effectIlaprazole
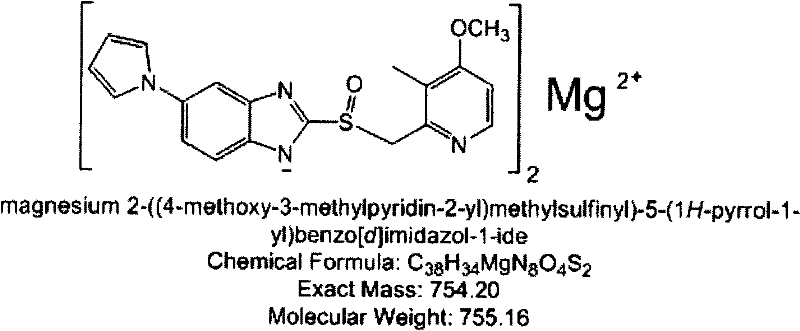
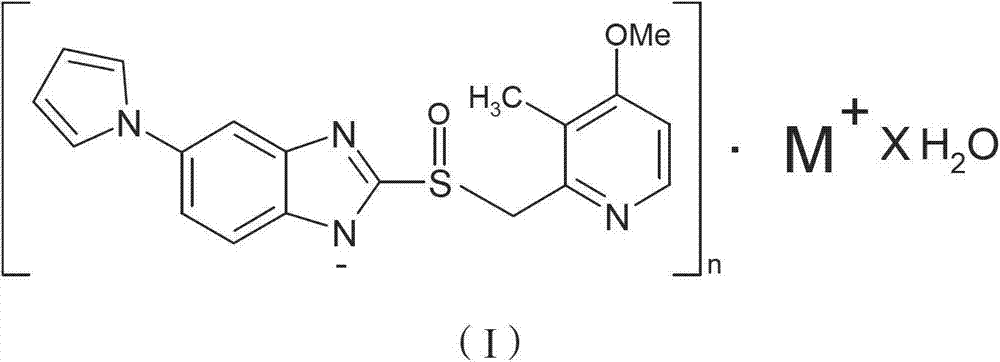
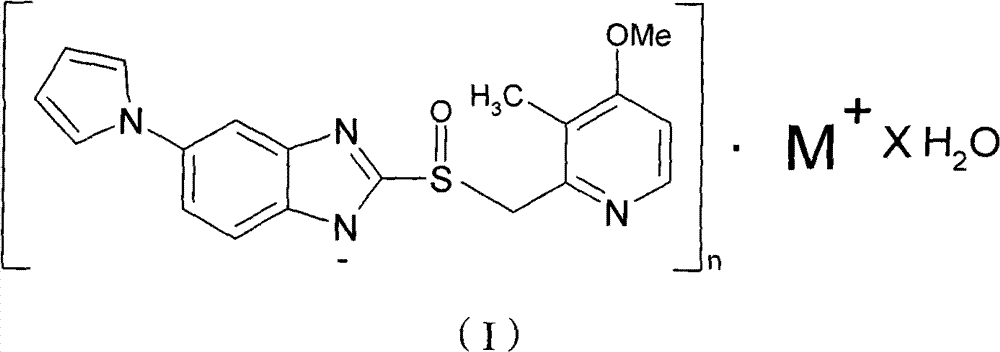
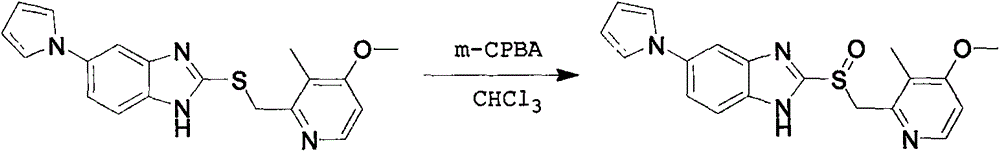
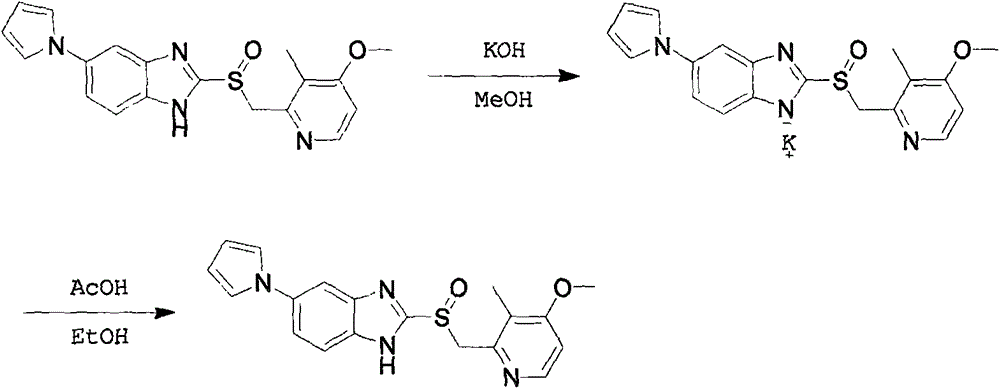
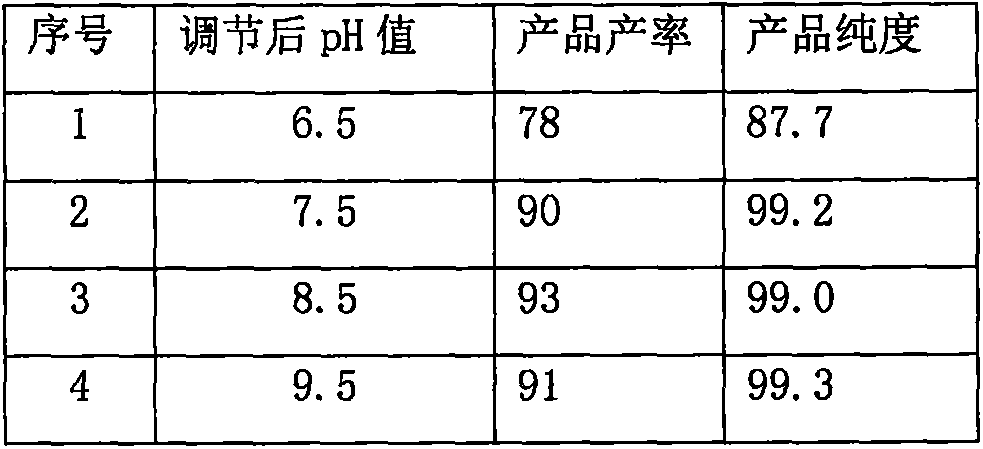
The invention provides a hydrate of an ilaprazole salt and a preparation method thereof. The hydrate is shown by a formula (I), wherein M is Li, Na or K, n is equal to 1, and x is equal to 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4 and 4.5 or M is Ca, Mg or Zn, n is equal to 2 and x is equal to 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4 and 4.5. The invention also provides application of the hydrate of the ilaprazole salt. Compared with ilaprazole and a salt thereof, the hydrate of the ilaprazole salt can be used as a proton pump inhibitor for treating diseases relevant with disorder in gastric acid secretion, has low side effect, and can be developed to form new clinically-acceptable medicaments. Simultaneously, the preparation method is simple and easy, and repeated experiments prove that the hydrate of the ilaprazole salt has a stable process and can be used for industrial production.
Owner:LIVZON PHARM GRP INC
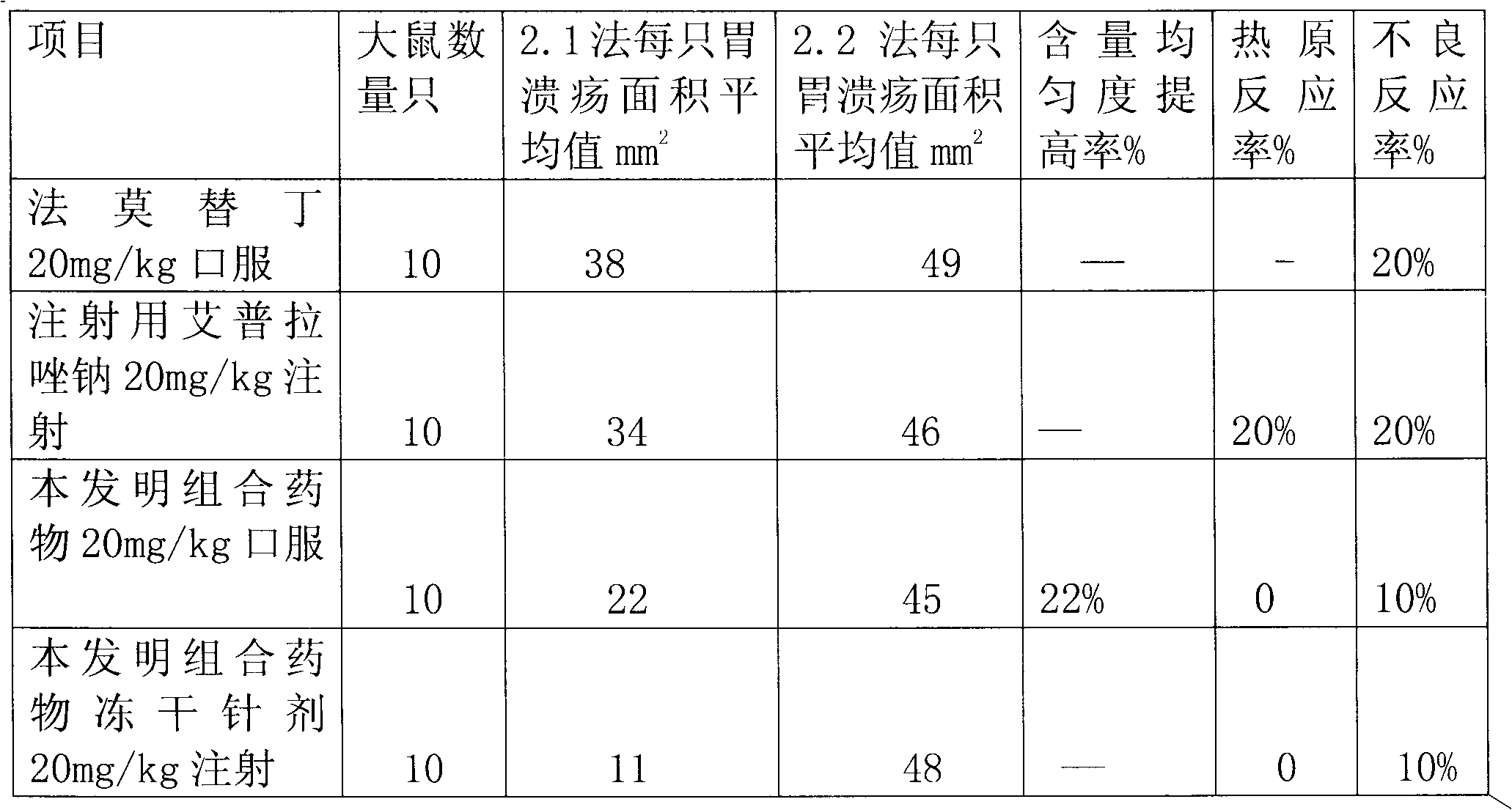
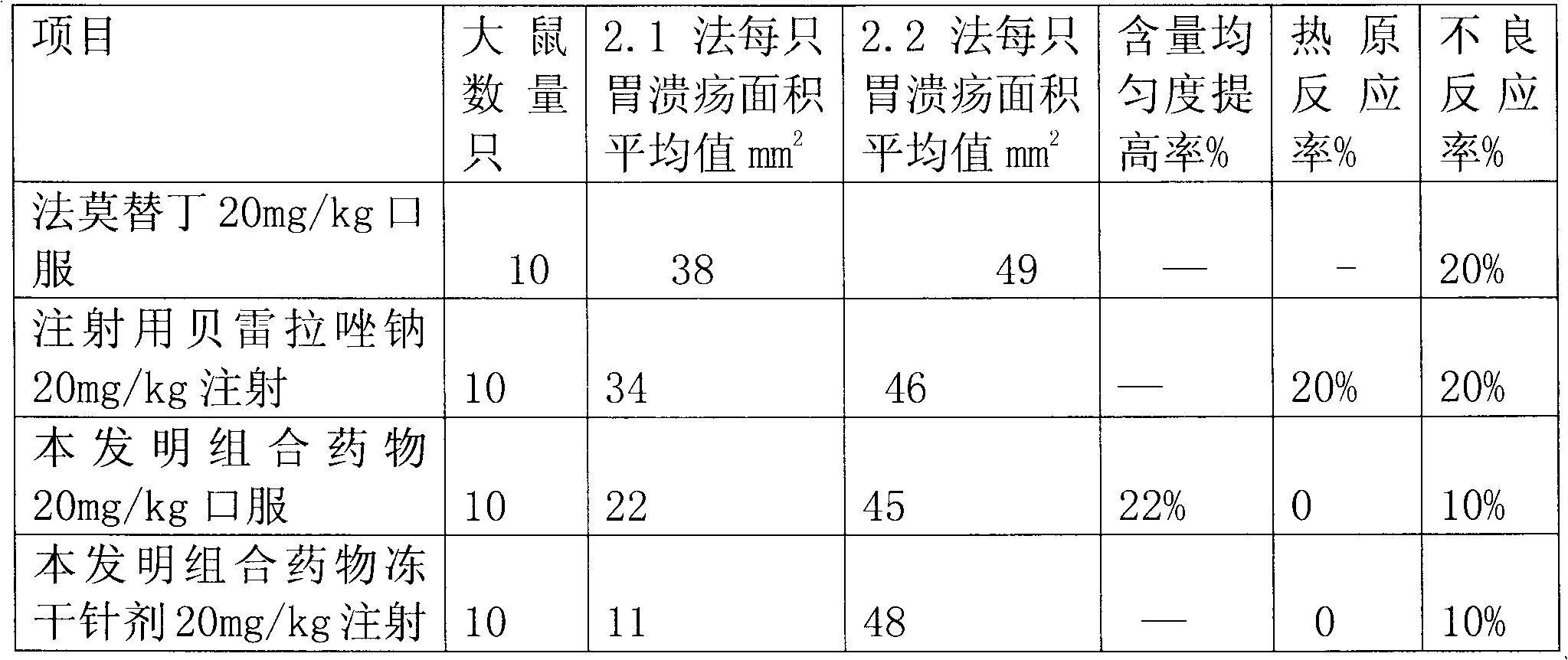
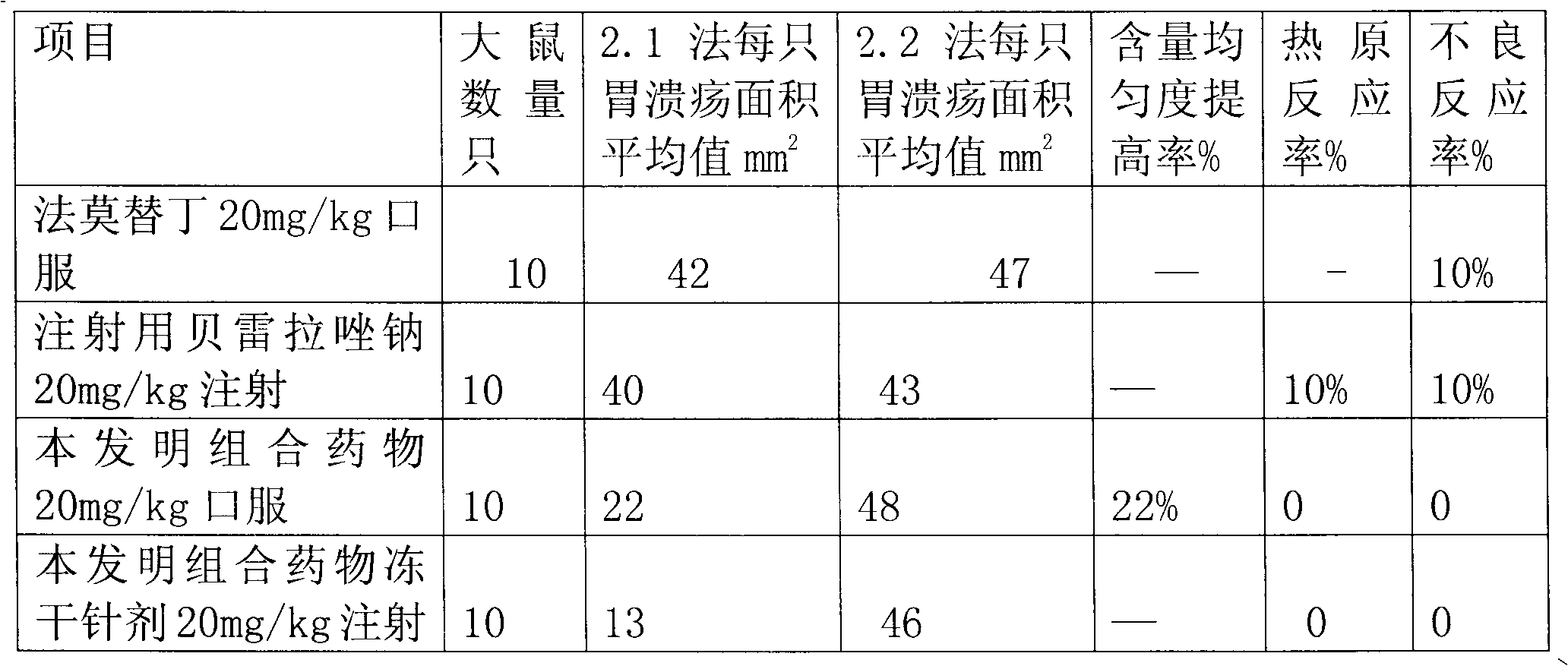
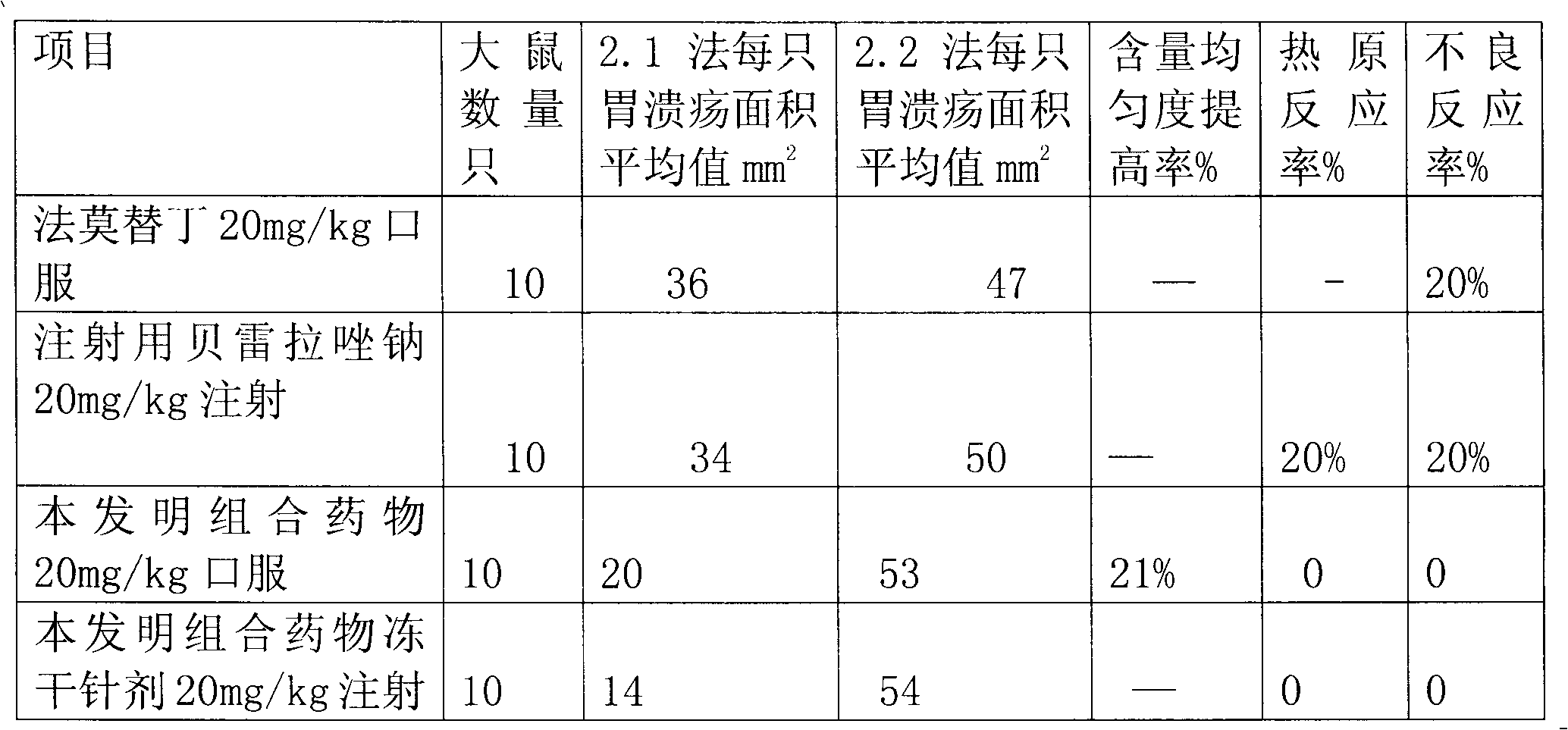
Combination medicament of ilaprazole sodium and preparation process thereof
InactiveCN102058593AEliminate adverse reactionsGood regeneration performanceOrganic active ingredientsDigestive systemCoated tabletsFreeze-drying
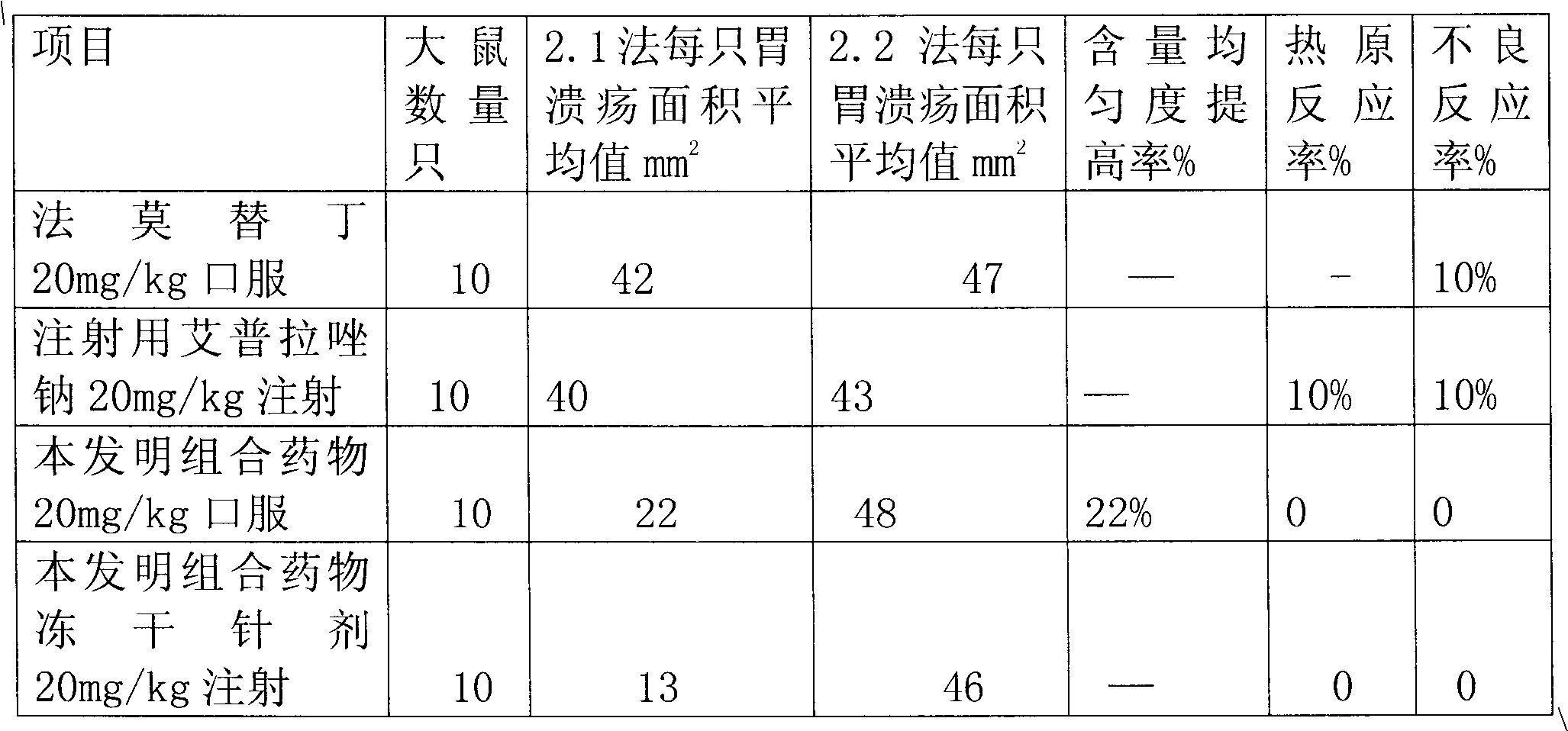
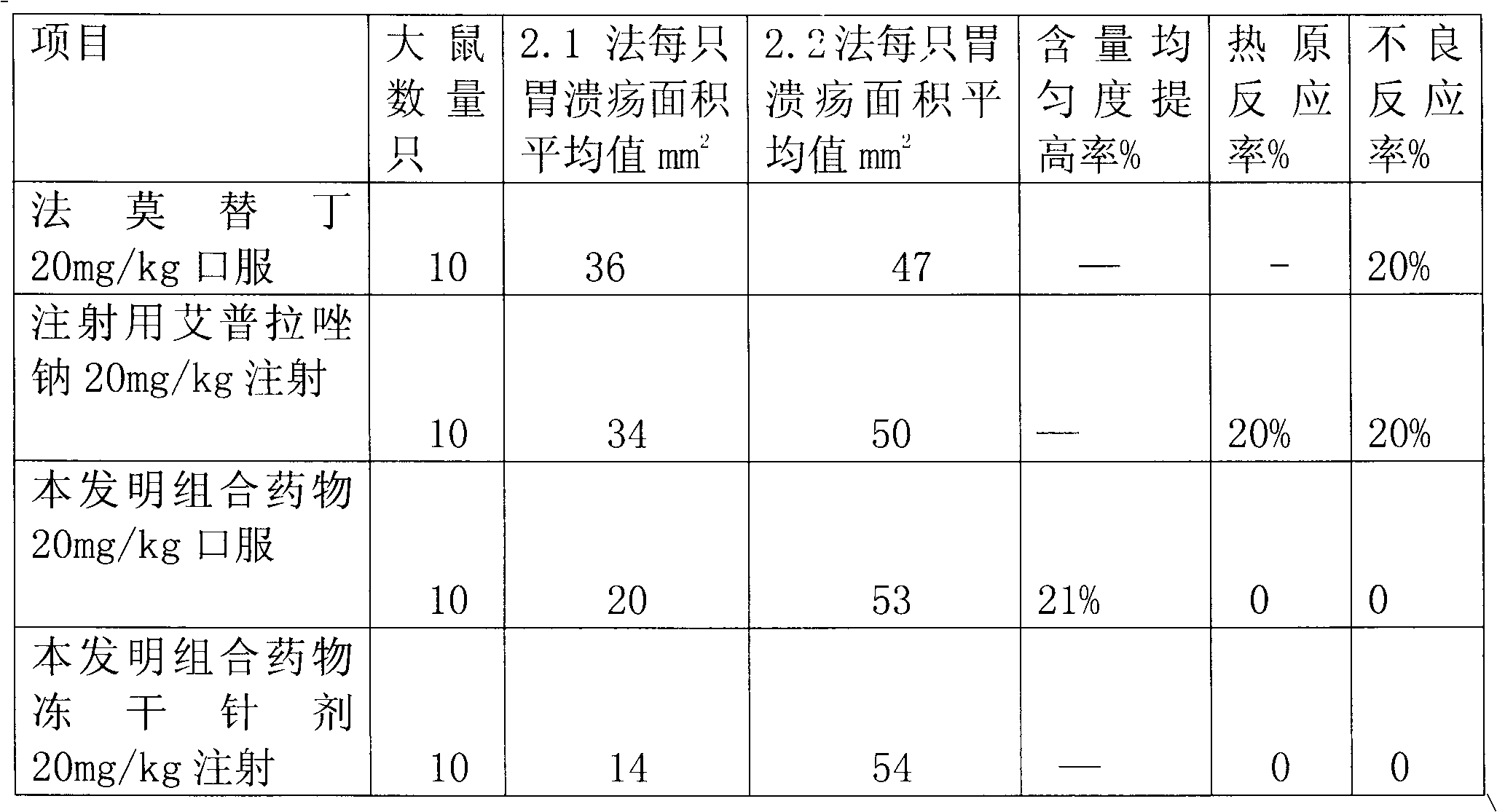
The invention provides a combination medicament of ilaprazole sodium. The medicament is characterized by comprising the following raw materials in percentage by weight: 5-10 percent of ilaprazole sodium, 25-35 percent of reduction composition containing glutathione and tiopronin with a weight ratio of 1:10 and 45-55 percent of diammonium glycyrrhizinate. The invention also provides a preparation process of the medicament. According to a pharmaceutically acceptable dosage of the ilaprazole sodium, the combination medicament can be respectively prepared into medical preparations of the dosage forms of injections of the combination medicament of the ilaprazole sodium, freeze-drying injections of combination medicament of the ilaprazole sodium, coated tablets of the combination medicament of the ilaprazole sodium, enteric capsules, sprays, and the like and is used for treating gastric ulcer.
Owner:吴赣英
Ilaprazole freeze-dried powder injection and preparation method thereof
ActiveCN103705476AGuaranteed reconstitution effectImprove securityOrganic active ingredientsPowder deliverySodium bicarbonateFreeze-drying
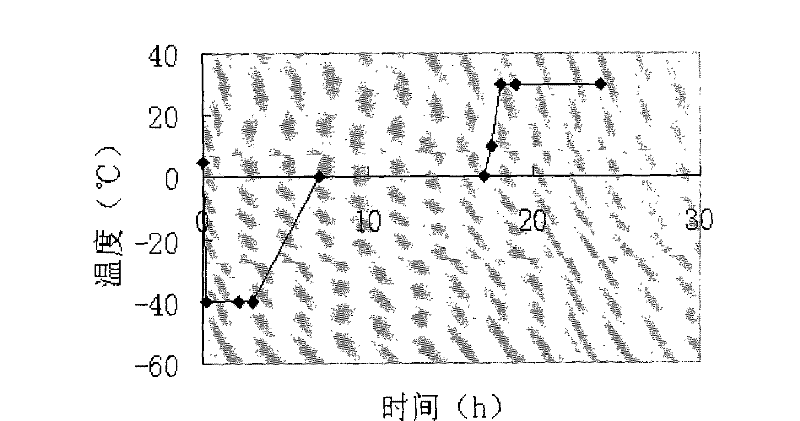
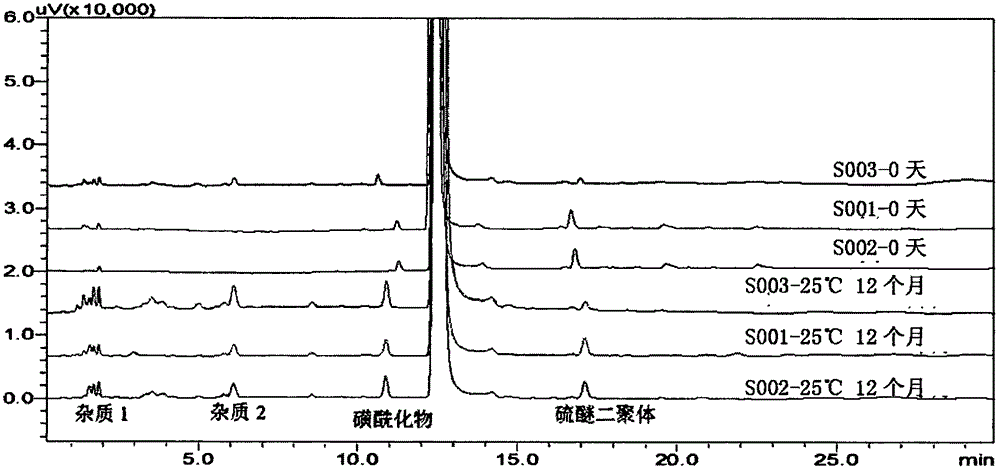
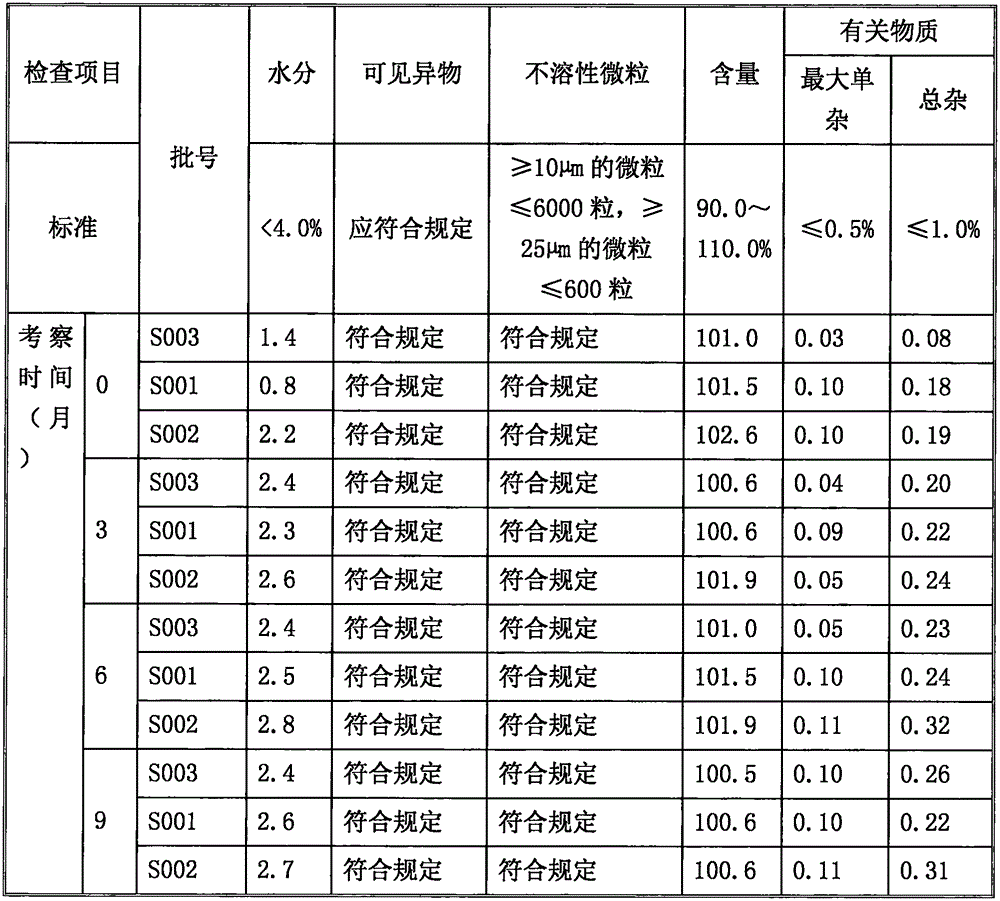
The invention relates to an ilaprazole freeze-dried powder injection and a preparation method thereof. The ilaprazole freeze-dried powder injection provided by the invention comprises an active ingredient consisting of ilaprazole or a pharmaceutically acceptable salt thereof, a stabilizer, a solubilizer and / or an excipient, wherein the solubilizer is an inorganic base, and the inorganic base is one or two or a composition of more than two of sodium hydroxide, potassium hydroxide, sodium bicarbonate and potassium bicarbonate; the stabilizer is one or two or a combination of meglumine and hydroxypropyl-beta-cyclodextrin. According to the ilaprazole freeze-dried powder injection provided by the invention, through adding the stabilizer and the solubilizer, the problem of poor stability of ilaprazole is solved, the solubility of ilaprazole is increased, and the ilaprazole freeze-dried powder injection is stable in quality and high in effect taking speed.
Owner:HARBIN SANLIAN PHARMA CO LTD
Sodium ilaprazole powder injection and preparation method thereof
The invention provides a sodium ilaprazole powder injection and a preparation method thereof. The powder injection is prepared from, by weight volume, 0.8-1.2% of sodium ilaprazole, 2.4-4.6% of mannitol and 0.05-0.15% of disodium edotato.
Owner:LIVZON GROUP LIVZON PHARMA FACTORY
Ilaprazole enteric coated tablet and preparation method thereof
InactiveCN102552190AImprove acid resistanceWidely distributedOrganic active ingredientsDigestive systemOrganic solventIlaprazole
The invention provides an ilaprazole enteric coated tablet and a preparation method thereof. The ilaprazole enteric coated tablet comprises enteric coated pellets and a pharmaceutically-acceptable tablet auxiliary material, wherein each enteric coated pellet comprises a pellet core, an isolating layer and an enteric coated layer; and the pellet core comprises ilaprazole or a pharmaceutically-acceptable salt thereof and a stabilizing agent. The enteric coated pellet tablet made from ilaprazole has high acid resistance; in a prescription, barrier substances such as an acid resisting agent, a surfactant, an organic solvent, a hydrophobic substance and the like are not contained, so that the health and safety of a human body are better facilitated; in the preparation method, an organic solvent is not used, so that operation is easy, and active substances are released quickly and stably; and moreover, the pellets in the tablet can be widely and uniformly distributed into an intestinal tract after administration, a dosage is poured out in a scatter way, and the distribution area of a medicament on the surface of the intestinal tract is increased, so that the stimulation of the medicament on the intestinal tract can be reduced or eliminated, and the bioavailability of the medicament is enhanced.
Owner:LIVZON PHARM GRP INC
Solid state forms of enantiopure ilaprazole
InactiveUS20080200515A1Inhibit gastric acid secretionInhibition is effectiveBiocideOrganic chemistryDiseaseIlaprazole
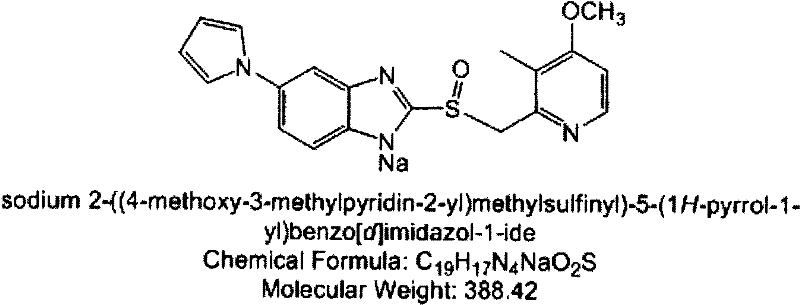
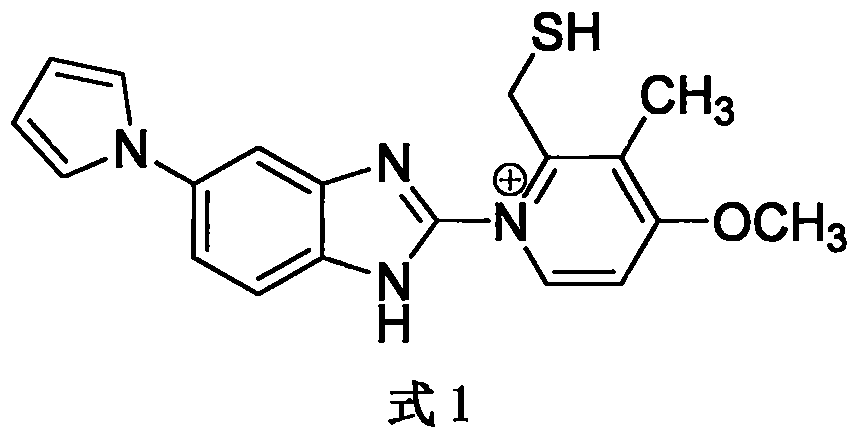
The invention relates to solid state forms of enantiopure ilaprazole, 2[[(4-methoxy-3-methyl-2-pyridinyl)-methyl]sulfnyl]-5-(1H-pyrrol-1-yl) 1H-Benzimidazole. The invention also relates to a pharmaceutical composition for inhibiting gastric acid secretion comprising a solid form of ilaprazole according to the invention in an amount effective to inhibit gastric acid secretion and a pharmaceutically acceptable carrier. The invention also provides methods of treatment for various acid-related gastrointestinal (GI) disorders such as those discussed above.
Owner:IL YANG PHARMA CO LTD
Sodium ilaprazole freeze-dried powder injection
ActiveCN105769777AStable in natureAdapt to clinical needsPowder deliveryOrganic active ingredientsDisodium EdetateMannitol
The invention aims at providing a sodium ilaprazole freeze-dried powder injection which has few adverse reactions and is stable in character and suitable for clinic requirements. The sodium ilaprazole freeze-dried powder injection is prepared from, by weight volume, 0.8-1.2% of sodium ilaprazole, mannitol and 0.05-0.15% of disodium edotato.
Owner:LIVZON GROUP LIVZON PHARMA FACTORY
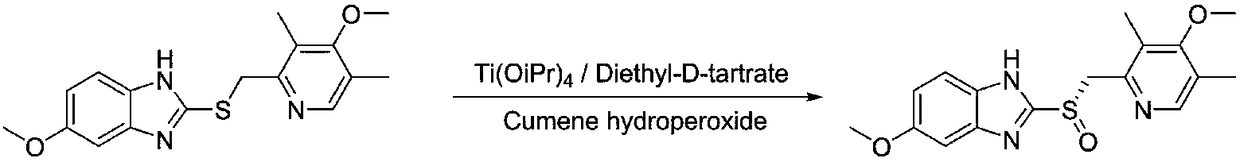
Preparation method of ilaprazole
The invention provides a preparation method of ilaprazole. According to the method, ilaprazole is prepared through a thioether intermediate; obtained ilaprazole is high in quality and has no overoxidation impurities; the method is simple and easy, high in yield, low in cost, environment-friendly, and suitable for industrial production, and has no need for special equipment; and no toxic chloroform solvent is used.
Owner:LIVZON PHARM GRP INC
Ilaprazole enteric capsule and preparation method thereof
InactiveCN102552256AImprove acid resistanceWidely distributedOrganic active ingredientsDigestive systemIrritationSurface-active agents
The invention provides an ilaprazole enteric capsule and a preparation method thereof. The ilaprazole enteric capsule comprises enteric pellets and capsule auxiliary materials accepted in pharmacy, wherein the enteric pellets include pellet cores, medicine carrying layers, isolation layers and enteric coating layers, and the medicine carrying layers comprise ilaprazole or salt and stabilizing agents accepted in pharmacy. The ilaprazole enteric capsule has good acid resistance, is favorable for human health and safety due to the fact that prescription doesn't contain obstacle materials of anti-acid agents, surface active agents, organic solvents, hydrophobic materials or the like. After the capsule is taken, the pellets can be widely and evenly distributed in intestinal tracts. Dosage is dumped in scatter mode, so that distribution area of medicine on the surface of the intestinal tracts is enlarged, irritation of the medicine on the intestinal tracts is reduced or eliminated, and biological utilization rate of the medicine is improved. When the capsule is taken, content in the capsule can be poured out to be directly taken. Thus, the capsule is also suitable for patients with difficulties in swallowing and infants.
Owner:LIVZON PHARM GRP INC
Sodium ilaprazole medicine composition freeze-dried powder injection for treating peptic ulcer
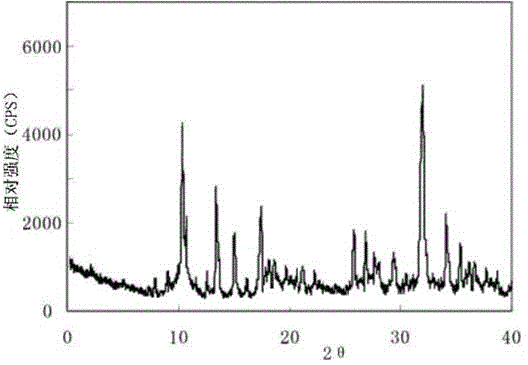
InactiveCN105055342AHigh purityImprove liquidityOrganic active ingredientsPowder deliveryPeptic ulcerPharmaceutical drug
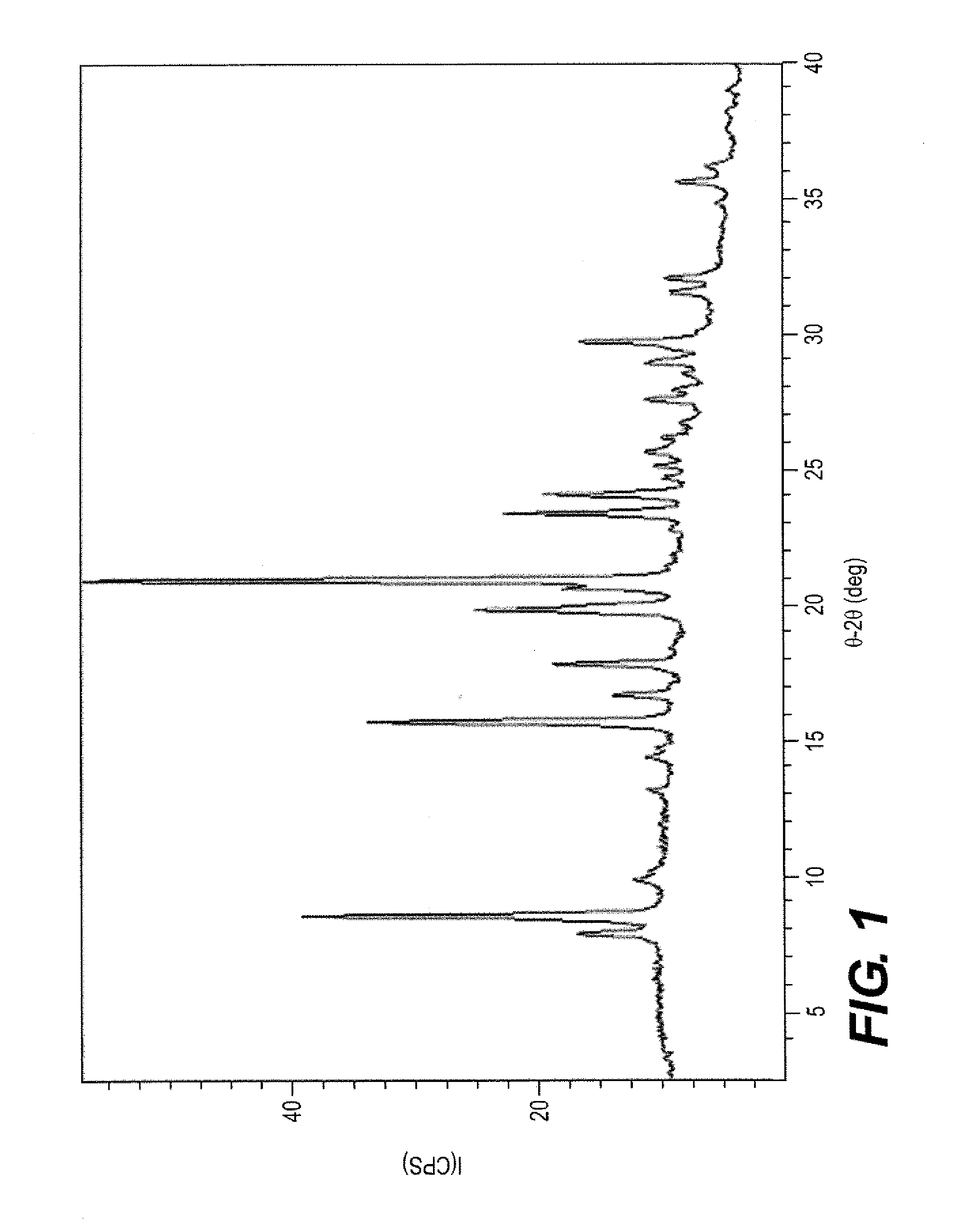
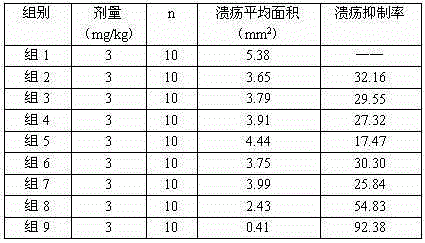
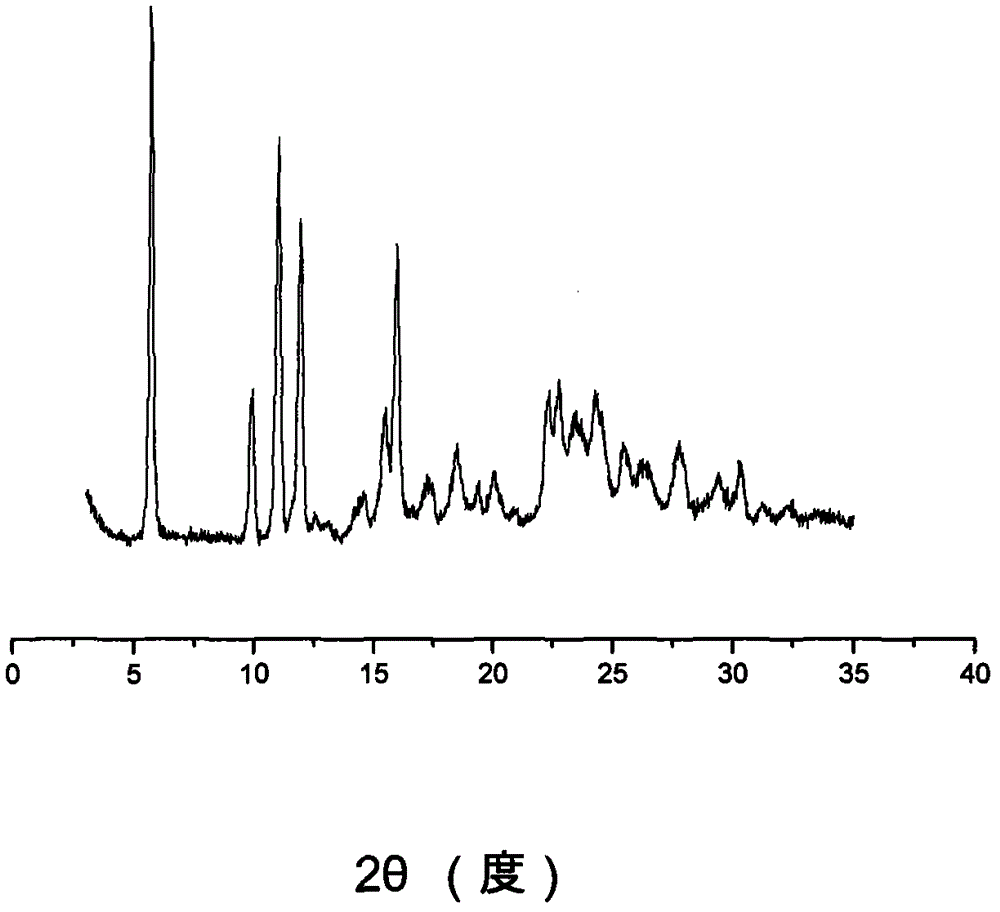
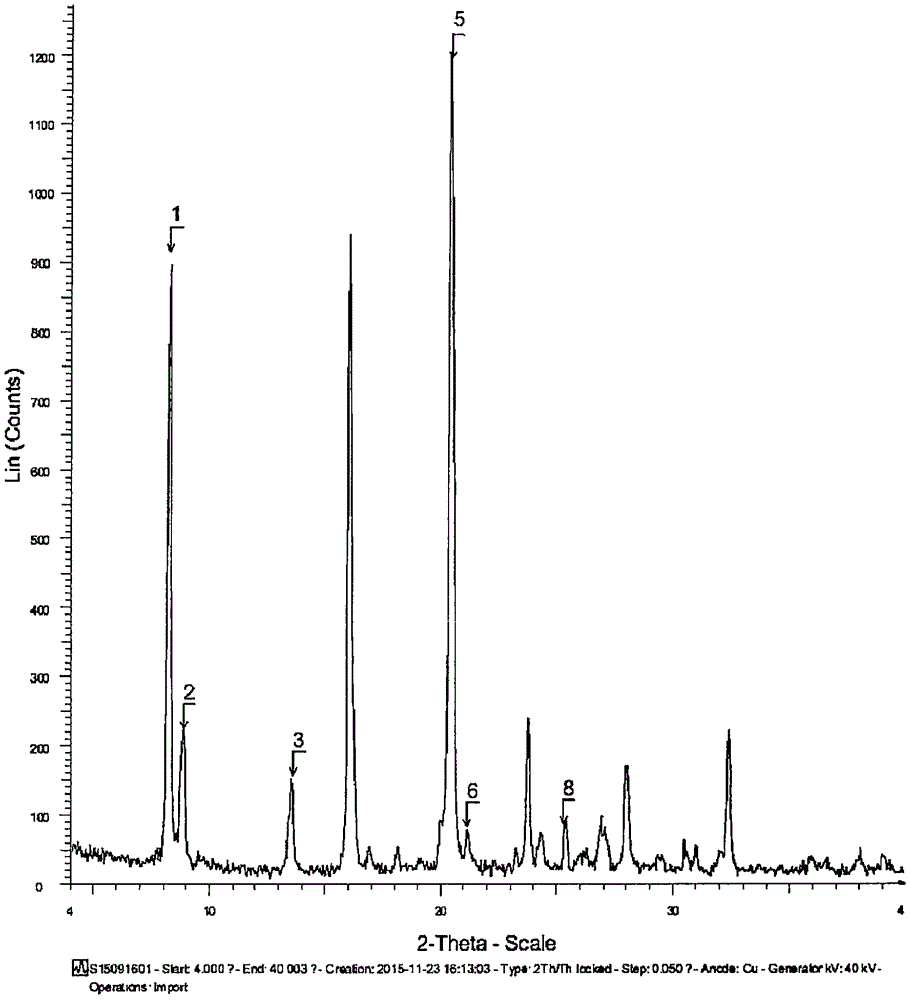
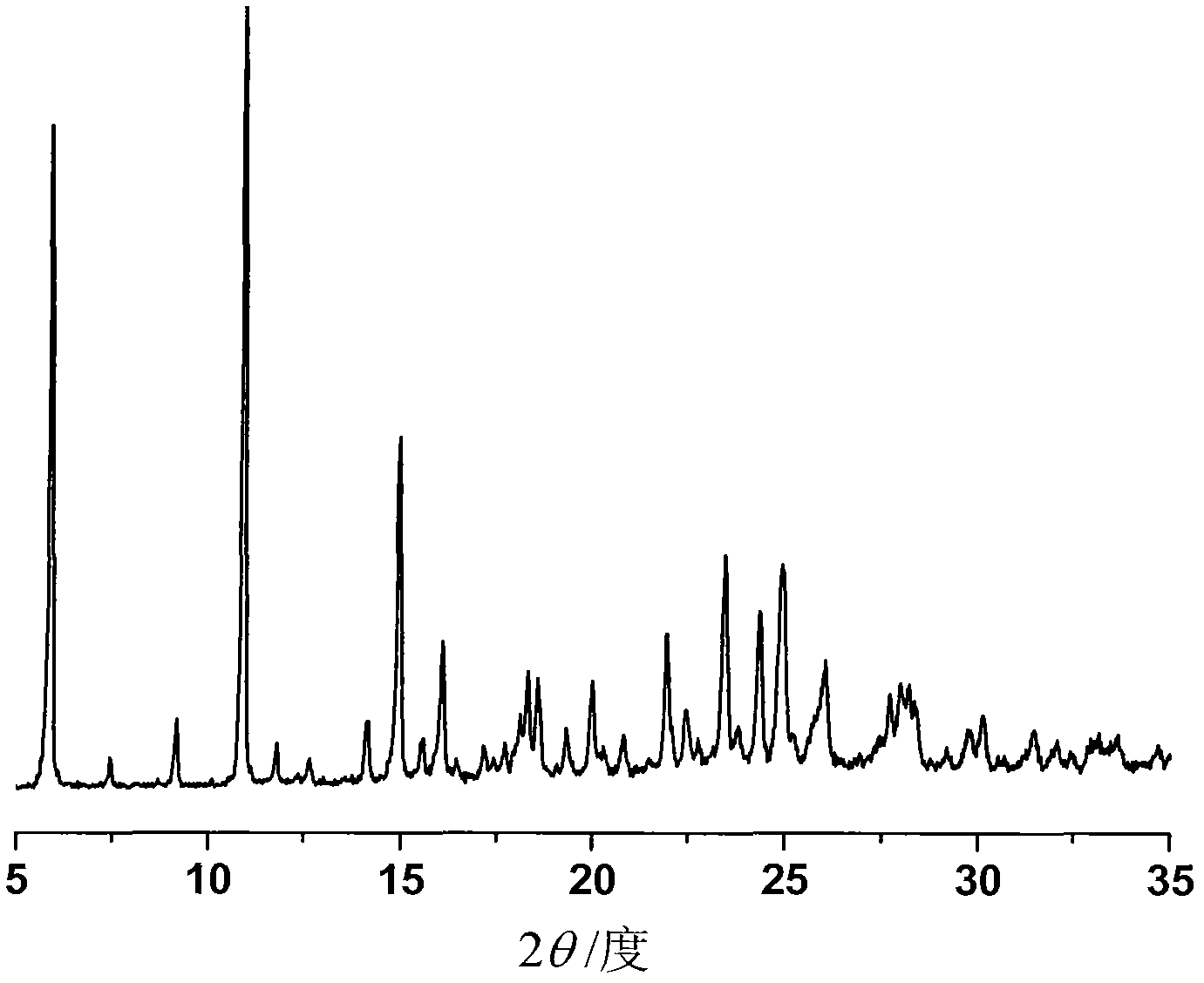
The invention discloses a sodium ilaprazole medicine composition freeze-dried powder injection for treating peptic ulcer, and belongs to the technical field of medicines. The sodium ilaprazole medicine composition freeze-dried powder injection comprises sodium ilaprazole and an excipient, wherein the excipient is low molecular dextran; sodium ilaprazole is a novel crystal compound different from that reported in the prior art, and the X-ray powder diffraction pattern of sodium ilaprazole is measured by utilizing Cu-K alpha rays and shown in Figure 1. Experiments prove that the novel crystal sodium ilaprazole compound is high in purity, liquidity and stability, low in impurity content and hygroscopicity, and safe and reliable in clinical application; the sodium ilaprazole medicine composition freeze-dried powder injection prepared by utilizing the novel crystal sodium ilaprazole compound is high in bioavailability and stability; the stability of the sodium ilaprazole medicine composition freeze-dried powder injection remains high after the sodium ilaprazole medicine composition freeze-dried powder injection is compatible with a solvent; the content of insoluble particles is extremely low. Therefore, the sodium ilaprazole medicine composition freeze-dried powder injection is particularly suitable for clinical application.
Owner:QINGDAO LANSHENGYANG PHARMA & BIOTECH CO LTD
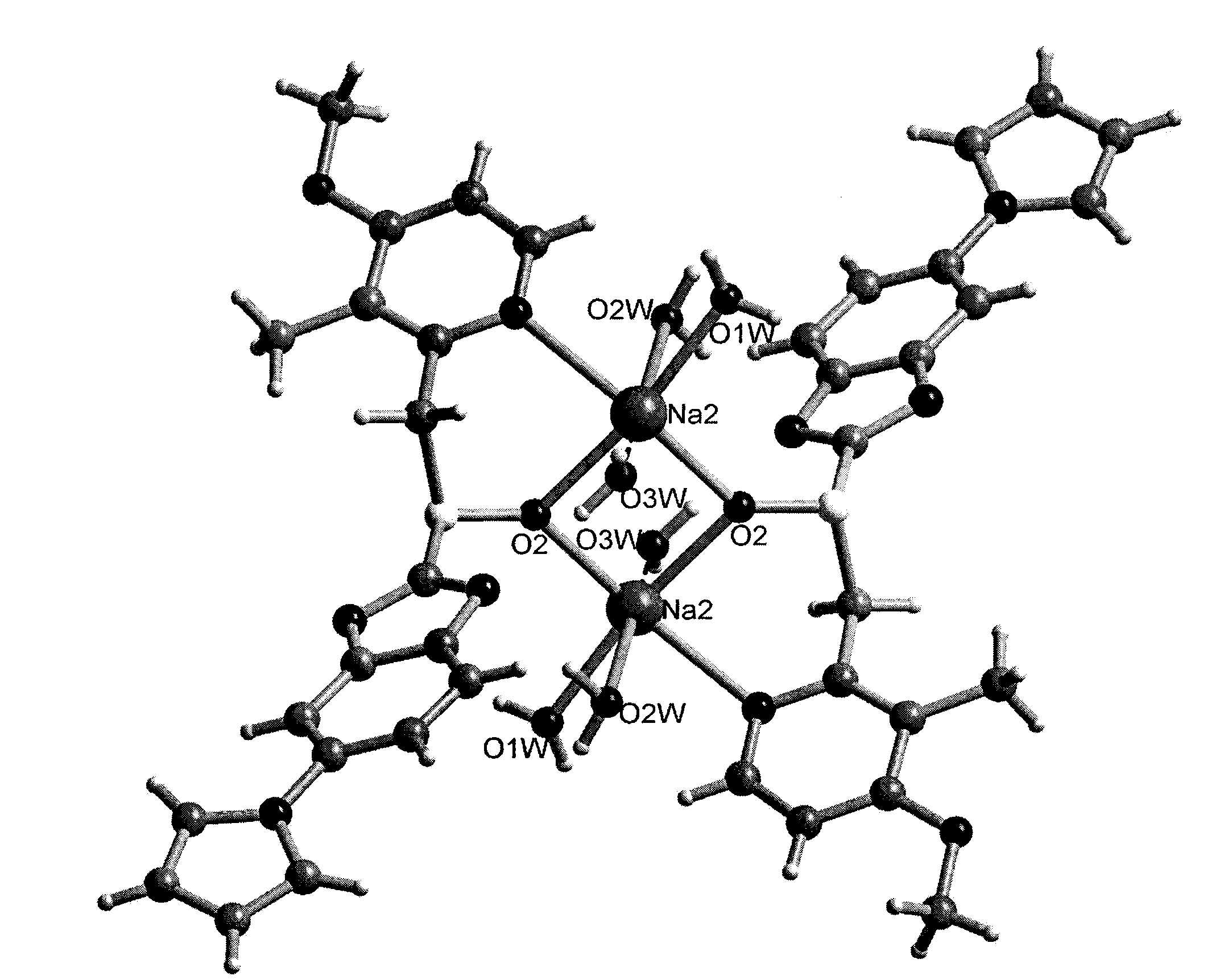
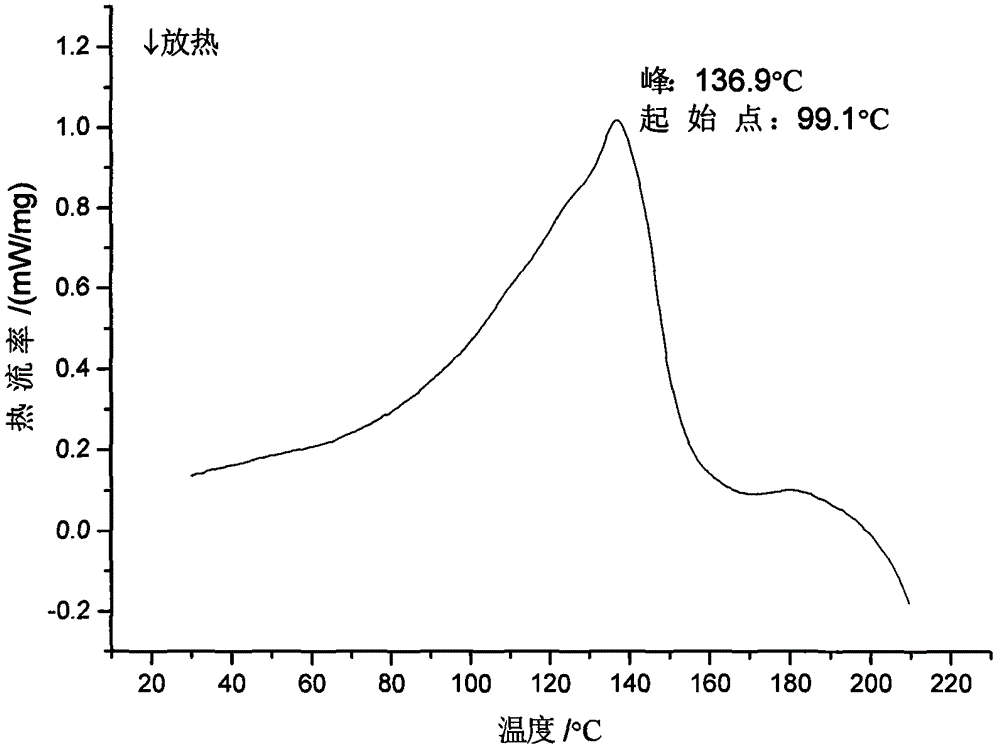
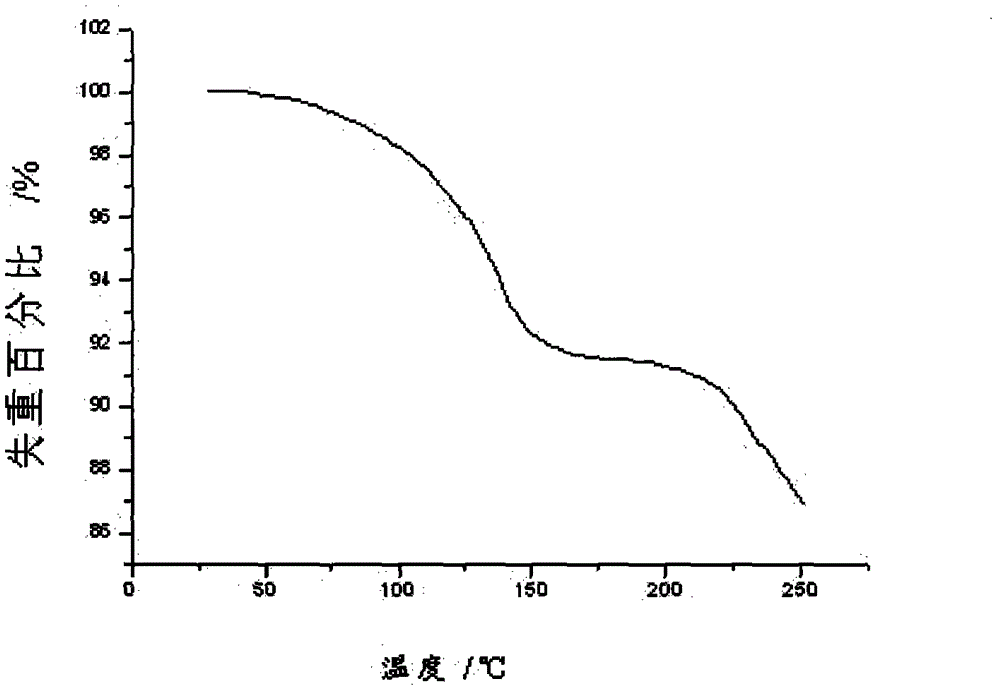
Crystalline Ilaprazole sodium hydrate and preparation method thereof
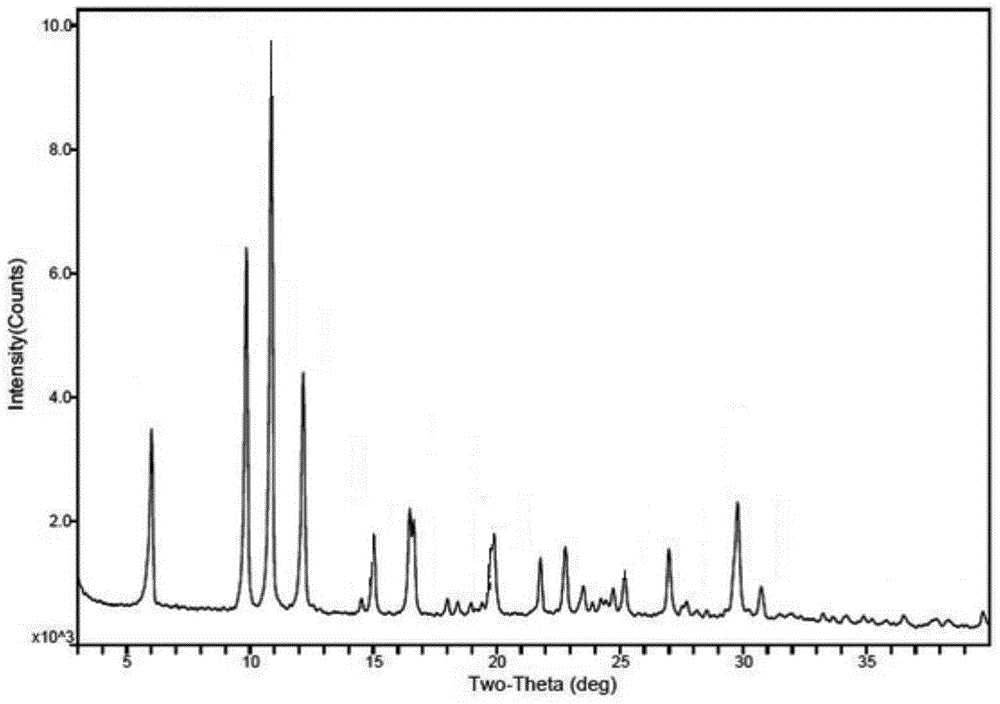
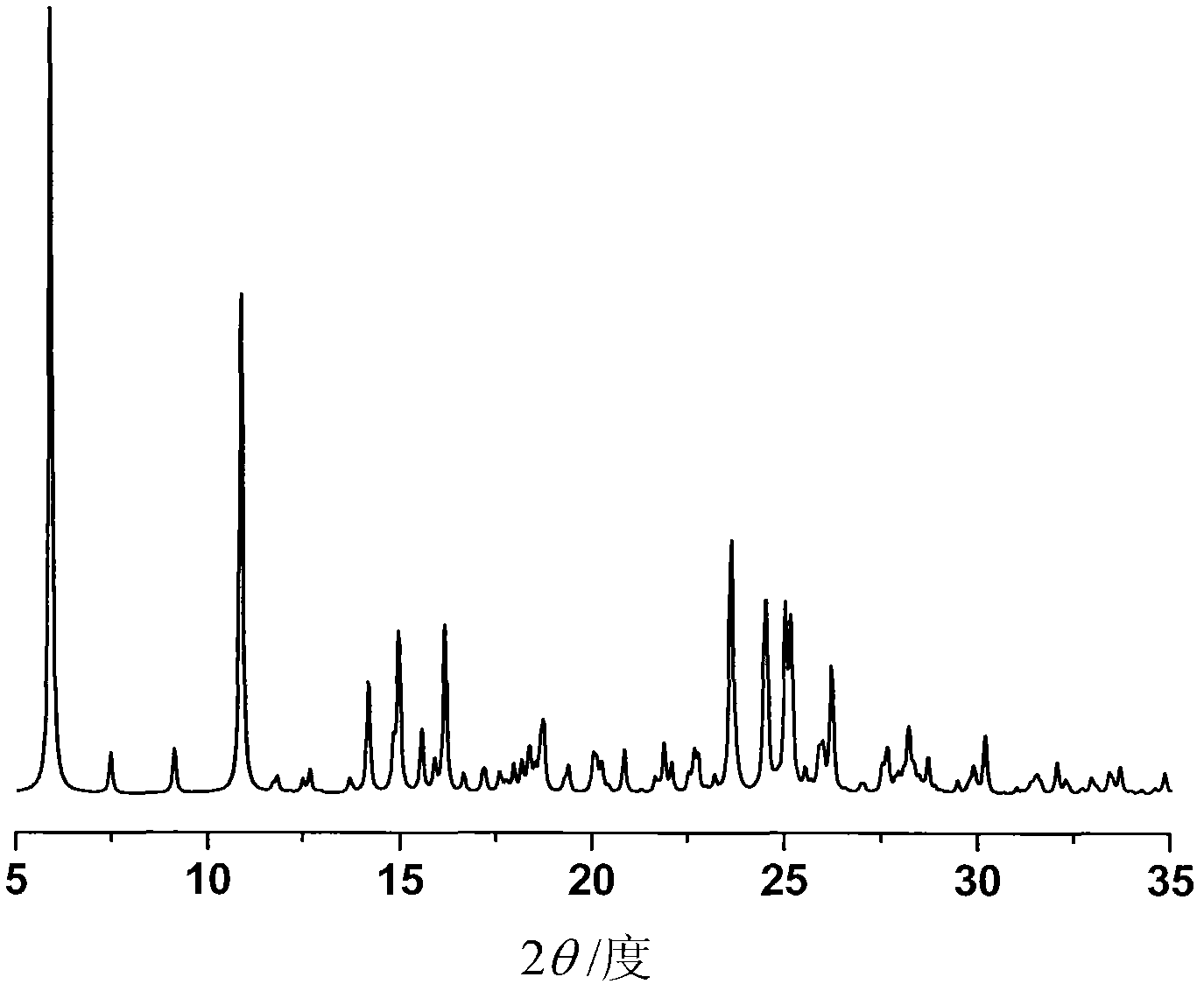
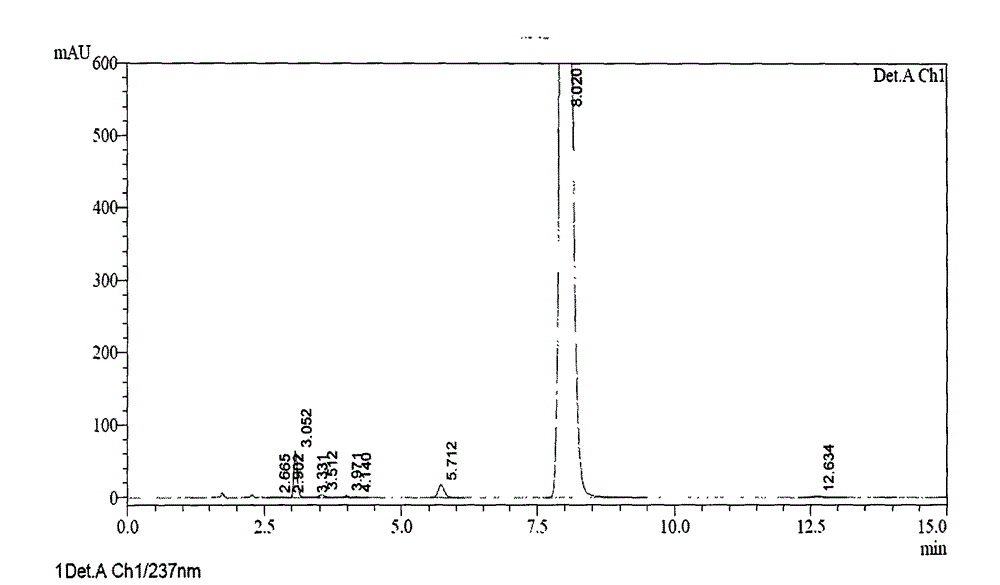
ActiveCN103204842AHigh crystal purityImprove bioavailabilityOrganic active ingredientsOrganic chemistryX-rayBioavailability
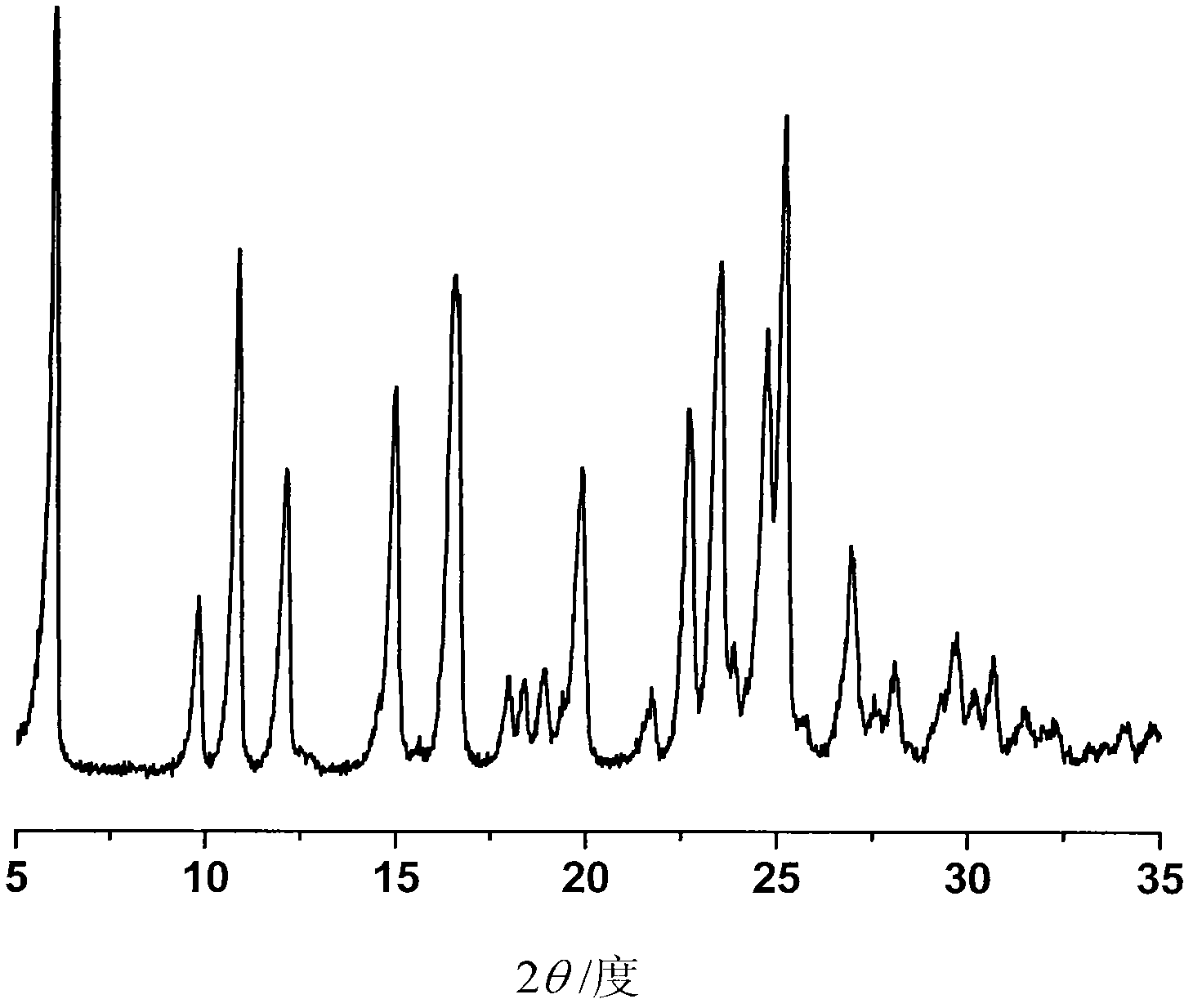
The invention provides a crystalline Ilaprazole sodium hydrate and a preparation method thereof. An X ray powder diffraction spectrum of the crystalline Ilaprazole sodium hydrate provided by the invention includes diffraction peaks represented by the following 2 theta angles: 5.9 degrees + / - 0.1 degree, 25.4 degrees + / - 0.1 degree and 10.7 degrees + / - 0.1degree. The crystalline Ilaprazole sodium hydrate provided in the invention has high crystal purity. In addition, the method provided in the invention for preparation of the crystalline Ilaprazole sodium hydrate has the advantages of simple operation, mild reaction conditions, easy control, low production cost, definite obtaining of a target product crystal with good repeatability, less introduction of impurities, and significantly improves drug bioavailability.
Owner:LIVZON PHARM GRP INC
Crystal-form ilaprazole sodium and preparation method thereof
The invention discloses a crystal-form ilaprazole sodium and a preparation method thereof. The novel ilaprazole sodium crystal form provided by the invention is easy to prepare, and has advantages of high purity and low impurity content. The preparation method provided by the invention needs low solvent amount, has advantages of low production cost, simple operation and mild reaction condition, is easy to control, and can produce the target product crystalline form doubtlessly and well-repeatedly.
Owner:LIVZON PHARM GRP INC
Ilaprazole sodium crystal form and preparation method thereof
The invention discloses an ilaprazole sodium crystal form and a preparation method thereof. The novel ilaprazole sodium crystal form provided by the invention is easy to prepare, and has advantages of high purity and low impurity content. The preparation method provided by the invention needs low solvent amount, has advantages of low production cost, simple operation and mild reaction condition, is easy to control, and can produce the target product crystalline form doubtlessly and well-repeatedly.
Owner:LIVZON PHARM GRP INC
Preparation method of ilaprazole
InactiveCN104650039AHigh yieldEasy to operateOrganic chemistryBiochemical engineeringProcess engineering
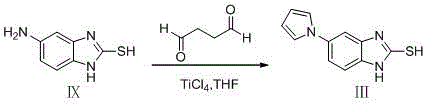
The invention belongs to the technical field of medicines and provides a preparation method of ilaprazole. The method comprises the following steps: by taking 2-nitry-1, 4-phenylenediamine as an initial raw material, preparing an important intermediate 5-(1H-pyrrole-1-yl)-2-mercapto benzimidazole through the route; and then abutting and oxidizing to obtain ilaprazole. The invention not only provides a novel synthetic route, but also provides a novel method for purifying ilaprazole. The synthetic route is relatively high in yield, simple to operate without special preparation and easy for industrial production. The purification method is simple to operate, and products are obtained by primary treatment without repeated purification, so that a lot of manpower and material resources and time are saved, and moreover, the product is high in purity and good in quality.
Owner:SHENYANG PHARMA UNIVERSITY
Method for synthesizing high antipode content benzimidazole derivative by unsymmetrical oxidizing thioether into sulphoxide
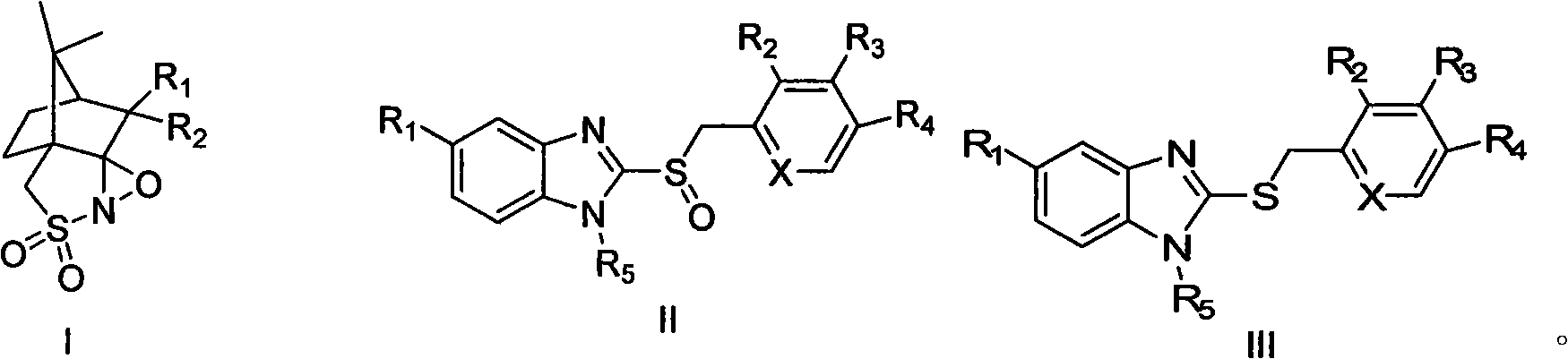
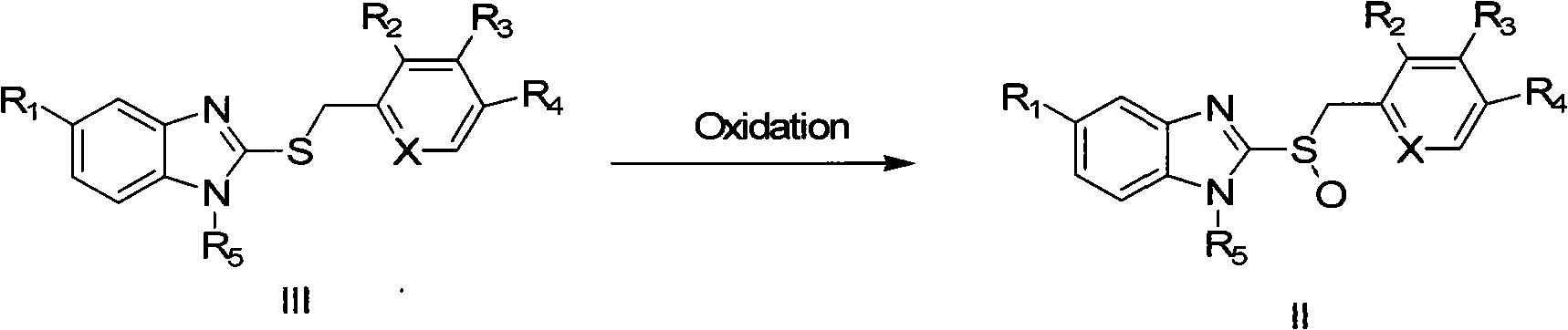
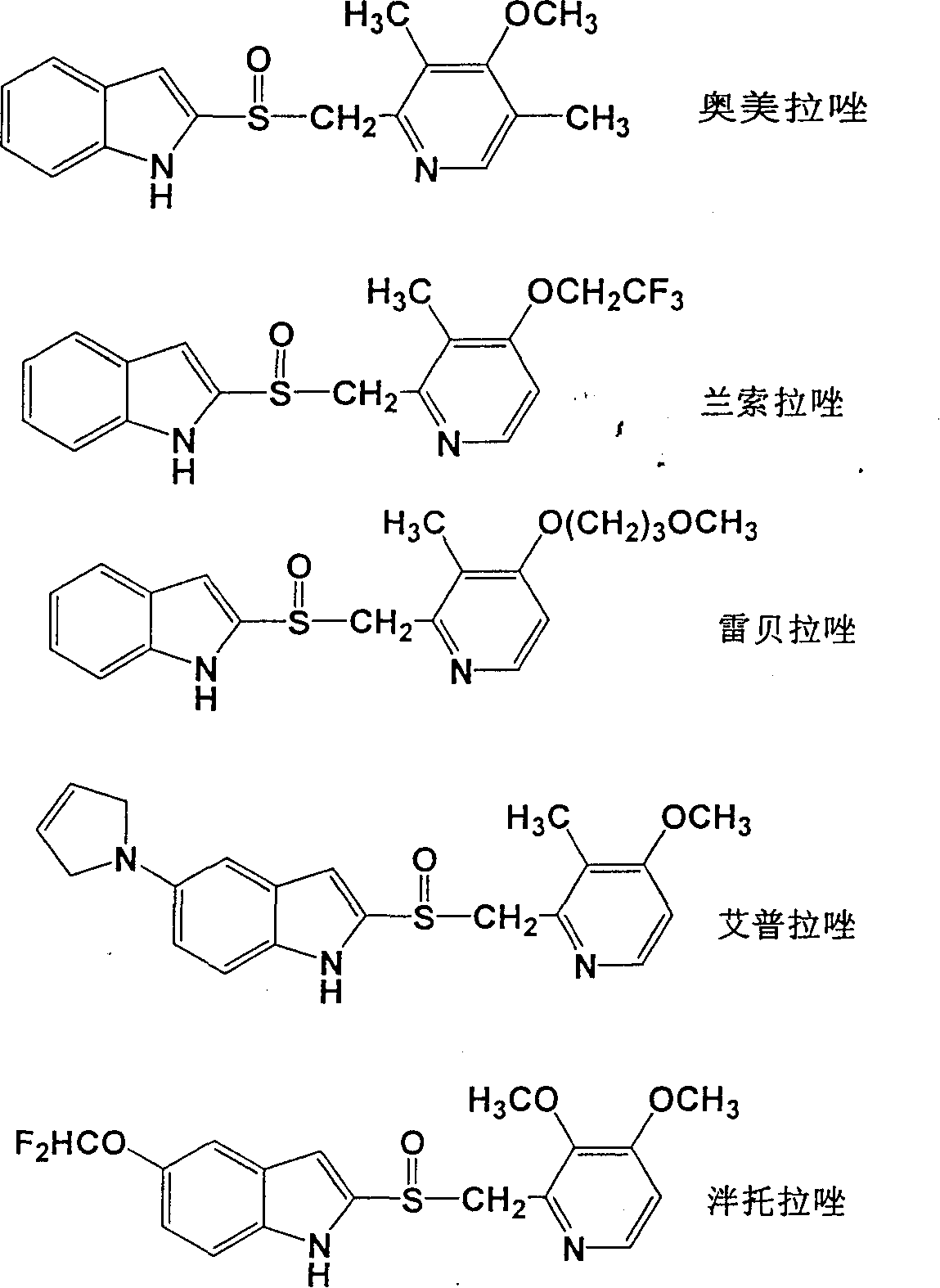
The invention discloses a new method for preparing razole compounds such as Omeprazole, Lansoprazole, Pailaiprazole, Leminoprazole, Ilaprazole, NC-1300-O-3 and 2-((4-methoxyl-6, 7, 8, 9-tetrahydro-5H-cyclohepta (b) pyridine-9-yl) sulfinyl)-1H-benzimidazole which are high enantiomorph content.
Owner:APELOA PHARM CO LTD +1
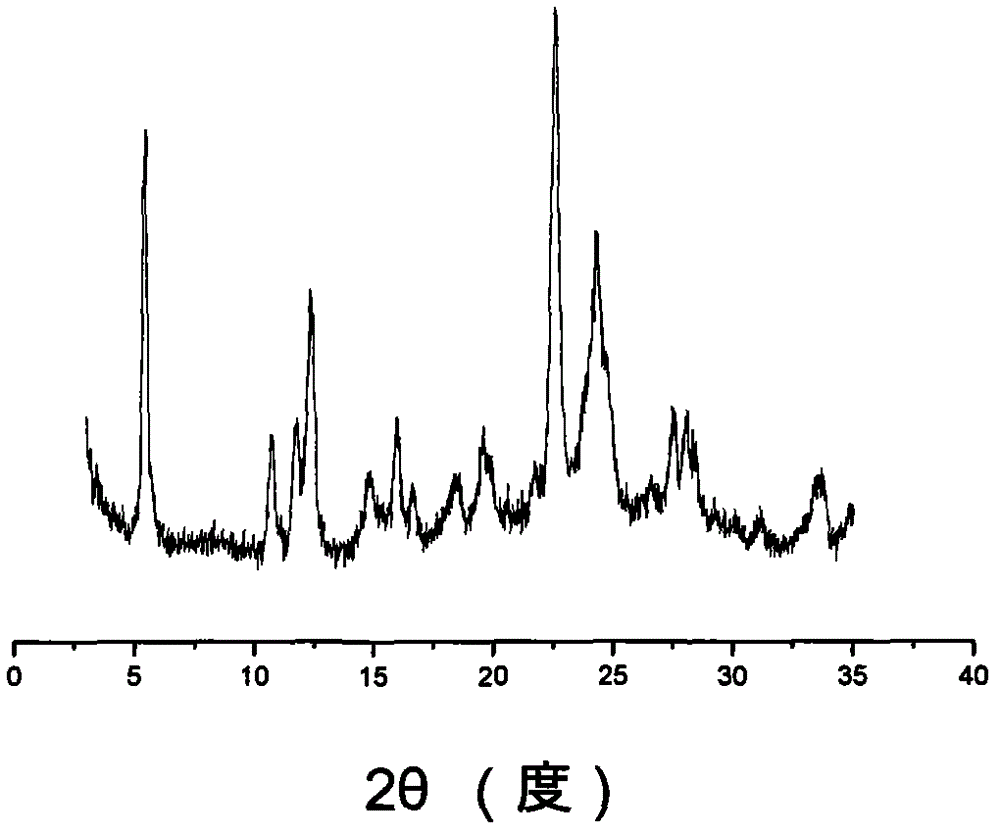
Ilaprazole crystal form II and preparation method thereof
The invention provides a novel Ilaprazole crystal form II. The novel Ilaprazole crystal form II is characterized in that an X-ray powder diffraction pattern has diffraction peaks at following 2-Theta degrees: 8.2+ / -1, 8.7+ / -1, 13.5+ / -1, 15.9+ / -1, 20.4+ / -1, 21.1+ / -1, 25.3+ / -1, 26.9+ / -1 and 28+ / -1. The crystal form is good in stability.
Owner:珠海保税区丽珠合成制药有限公司
Ilaprazole sodium compound and pharmaceutical composition thereof
InactiveCN105461692ACrystal stableImprove stabilityOrganic active ingredientsOrganic chemistryIlluminanceCombinatorial chemistry
The invention provides various crystal forms of an ilaprazole sodium compound. In tests with influence factors of illuminance, high temperature and high moisture and an acceleration test, the crystal forms are good in stability, wherein the crystal form C is more stable and is suitable for preparing injections and enteric solid preparations. The various crystal forms are simple in preparation method and are suitable for industrial production.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Ilaprazole freeze-dried composition
ActiveCN105982866AImprove complianceReduce moisture contentPowder deliveryOrganic active ingredientsFreeze-dryingMedicine
The invention provides a freeze-dried composition of ilaprazole and a preparation method of the freeze-dried composition. The ilaprazole freeze-dried composition, which is prepared in accordance with the method provided by the invention, can be kept stable in an environment which is relatively low in pH value, so that the compliance of clinical application is improved.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Improved preparation and separated purification method of benzimidazole type proton pump inhibitors and precursor thereof
ActiveCN100393712CSimple post-processingGood reproducibilityOrganic chemistryLansoprazolePurification methods
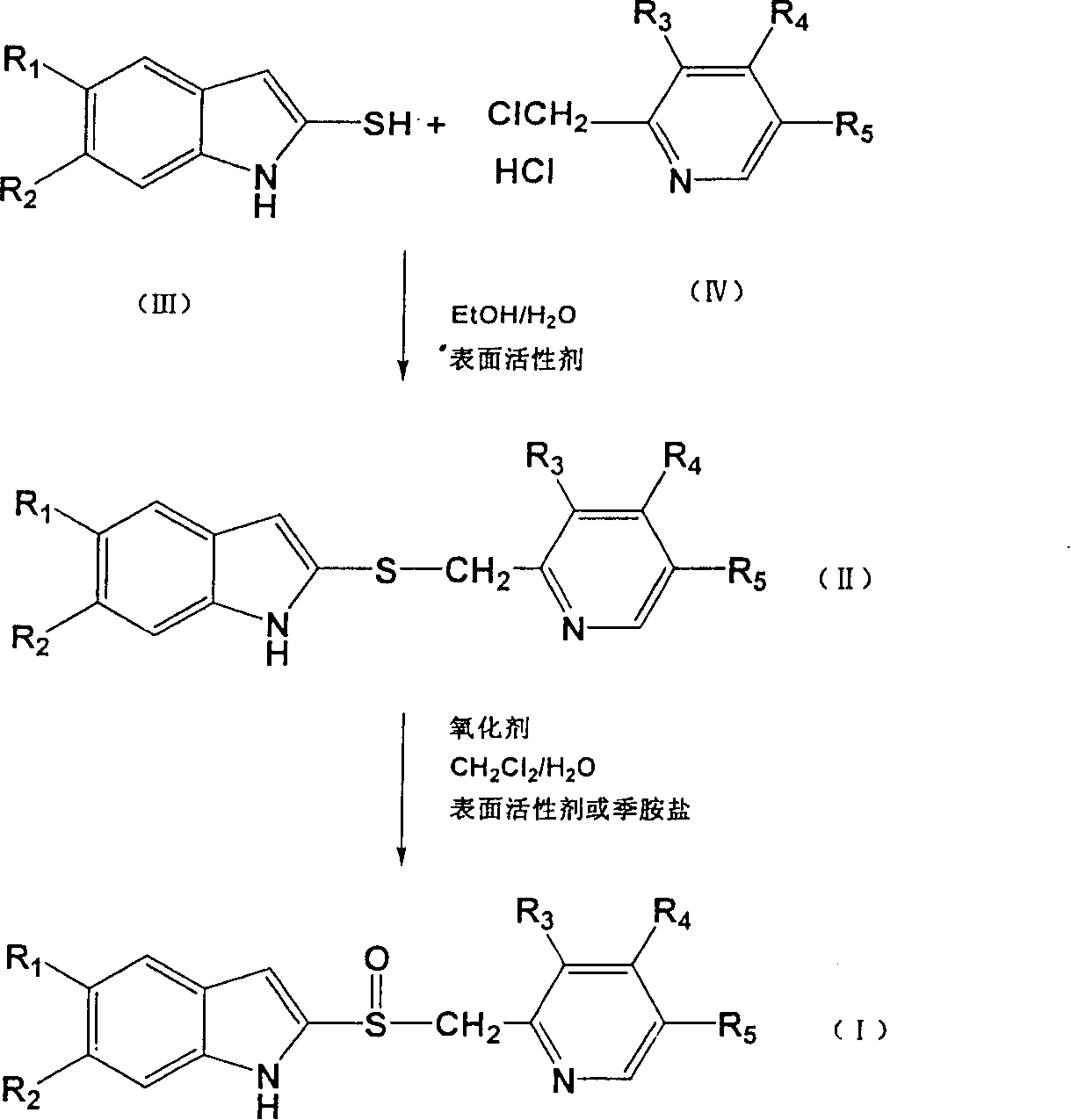
The provided preparation, separation and purification method for benzimidazole-type proton pump inhibitor and its precursor, such as omeprazole, rabeprazole, lansoprazole, and ilaprazole comprises: for the first time, adding surfactant or phase-transfer catalyst in the synthesis process to speed up and promote the reaction more complete. This invention has well reaction efficiency and selectivity to overcome the defects in prior art and benefit to post-treatment and crystallization.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI +1
Hydrate of ilaprazole salt, preparation method thereof and application thereof
ActiveCN102140092BGood treatment effectLittle side effectsOrganic active ingredientsOrganic chemistryDiseaseSide effect
Owner:LIVZON PHARM GRP INC
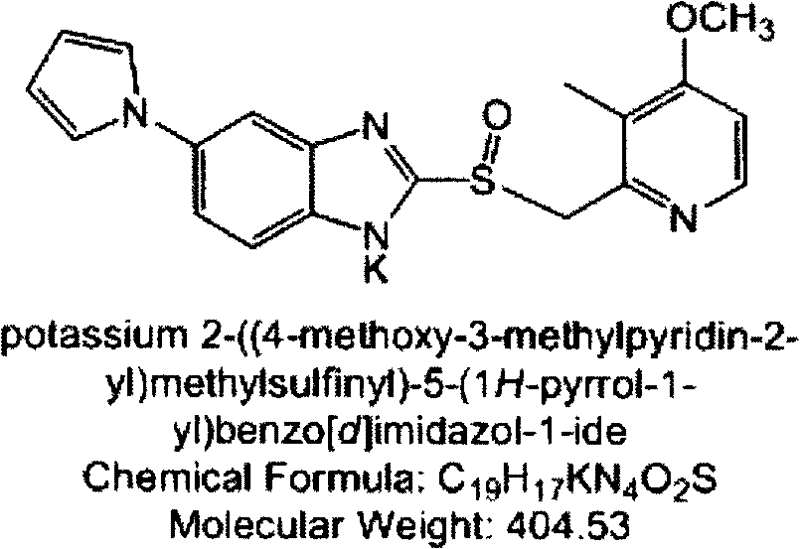
A preparing method for high-purity ilaprazole sodium
InactiveCN106045975AEfficient removalNo pollution in the processOrganic chemistryOrganic solventSolvent
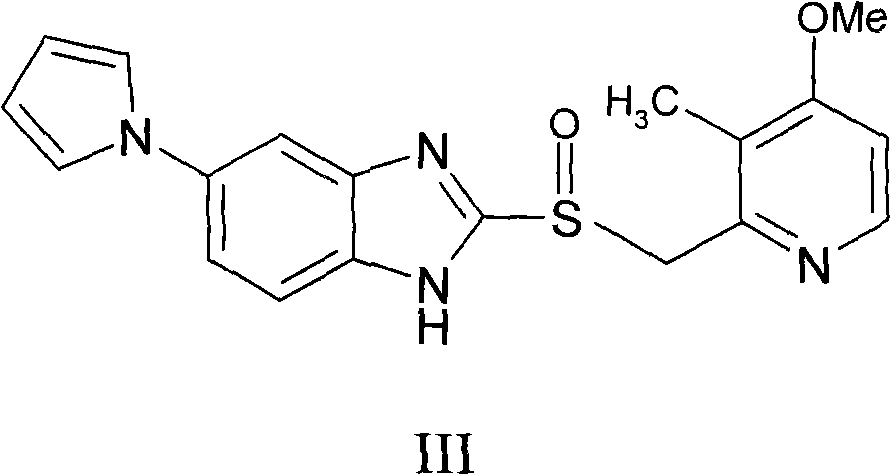
A preparing method for high-purity ilaprazole sodium is provided. The method includes 1) dissolving a 2-[(4-methoxy-3-methylpyridin-2-yl)methyl]sulfinyl-5-(1H-pyrrol-1-yl)-1H-benzimidazole crude product into an organic solvent, adding an inorganic alkali to form a salt, and filtering to obtain a 2-[(4-methoxy-3-methylpyridin-2-yl)methyl]sulfinyl-5-(1H-pyrrol-1-yl)-1H-benzimidazole salt (A), 2) dissolving the A into an organic solvent, adjusting pH with an acid until reaching alkalescence to prepare high-purity 2-[(4-methoxy-3-methylpyridin-2-yl)methyl]sulfinyl-5-(1H-pyrrol-1-yl)-1H-benzimidazole (B), and 3) reacting the prepared B with a sodium-containing compound in a water-containing solvent to prepare the ilaprazole sodium.
Owner:LIVZON PHARM GRP INC +1
Crystalline Ilaprazole sodium ethylate and preparation method thereof
ActiveCN103204843AHigh crystal purityImprove bioavailabilityOrganic chemistryDigestive systemX-rayIlaprazole
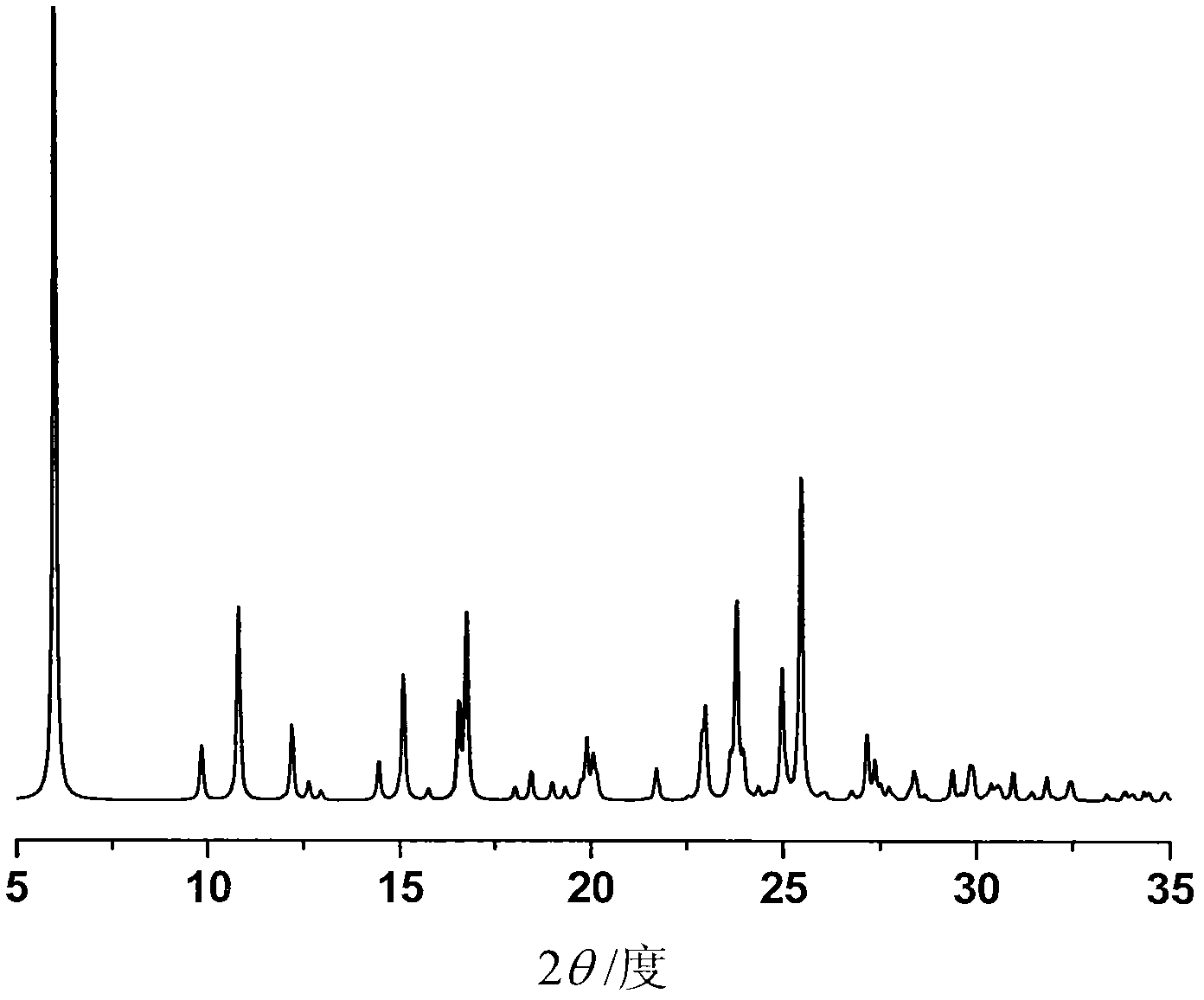
The invention provides a crystalline Ilaprazole sodium ethylate and a preparation method thereof. The X ray powder diffraction spectrum of the crystalline Ilaprazole sodium ethylate provided by the invention includes diffraction peaks represented by the following 2 theta angles: 5.9 degrees + / - 0.1 degree, and 10.8 degrees + / - 0.1 degree. The crystalline Ilaprazole sodium ethylate provided by the invention has high crystal purity. In addition, the method provided in the invention for preparation of the crystalline Ilaprazole sodium ethylate has the advantages of simple operation, mild reaction conditions, easy control, low production cost, definite obtaining of the target product crystal with good repeatability, fewer introduced impurities, and significantly improves the bioavailability of drugs.
Owner:LIVZON PHARM GRP INC
Rabeprazole sodium combined medicament and preparation process thereof
InactiveCN102091070AEliminate adverse reactionsGood regeneration performanceOrganic active ingredientsDigestive systemDrugs preparationsRabeprazole Sodium
The invention provides a rabeprazole sodium combined medicament and a preparation process thereof. The rabeprazole sodium combined medicament is characterized by comprising the following raw materials as components in proportion by weight: 5-10 of ilaprazole sodium, 20-30 of composition of reduced glutathione and hepatocyte growth-promoting factors in proportion by weight of 1:10, and 50-60 of diammonium glycyrrhizinate. According to a pharmaceutically allowable dose of rabeprazole sodium, pharmaceutical preparations in dosage forms of injection, lyophilized powder injection, enteric coated tablets, enteric coated capsules, spray and the like of the rabeprazole sodium combined medicament are respectively prepared for treating gastric ulcer.
Owner:吴赣英
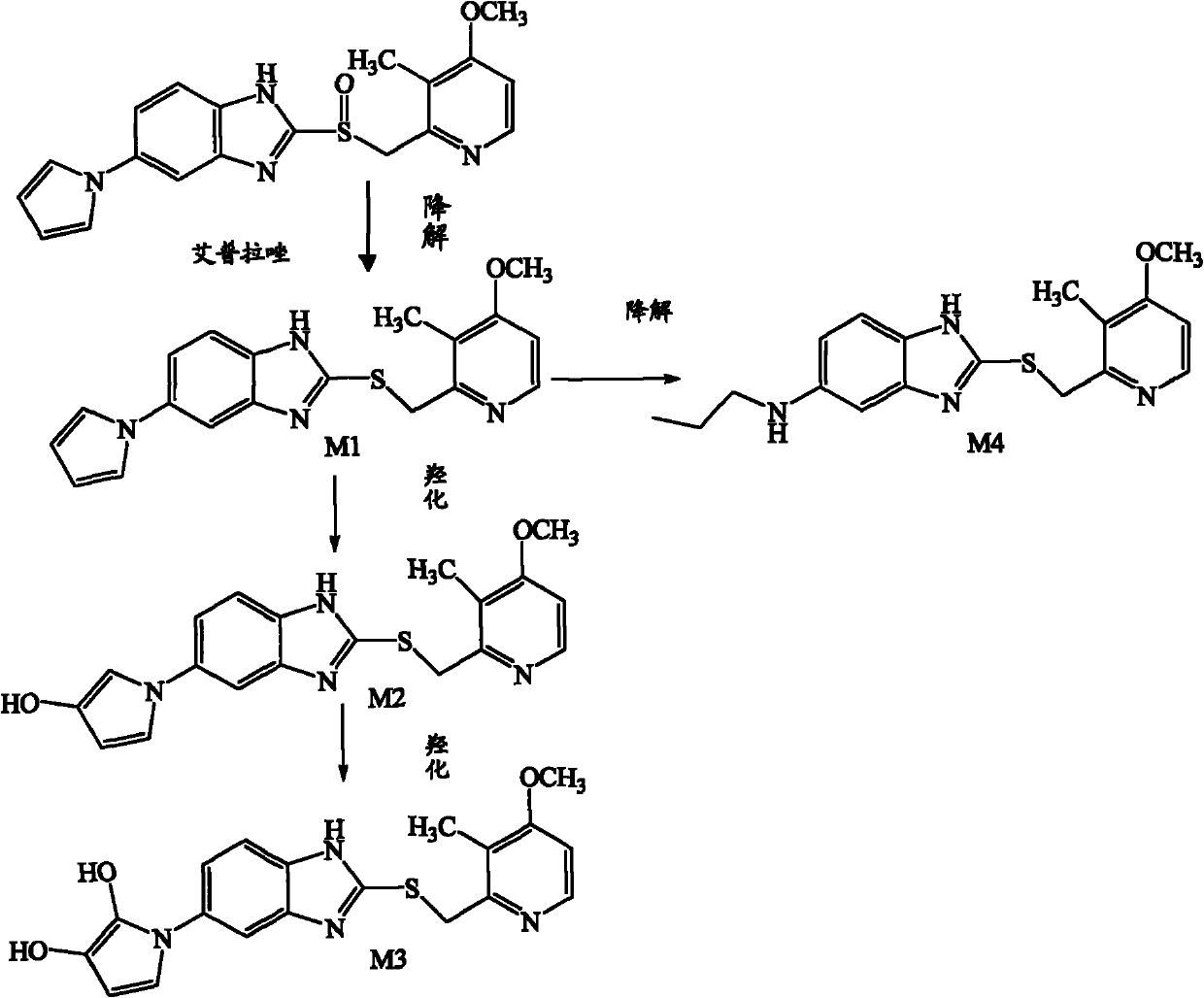
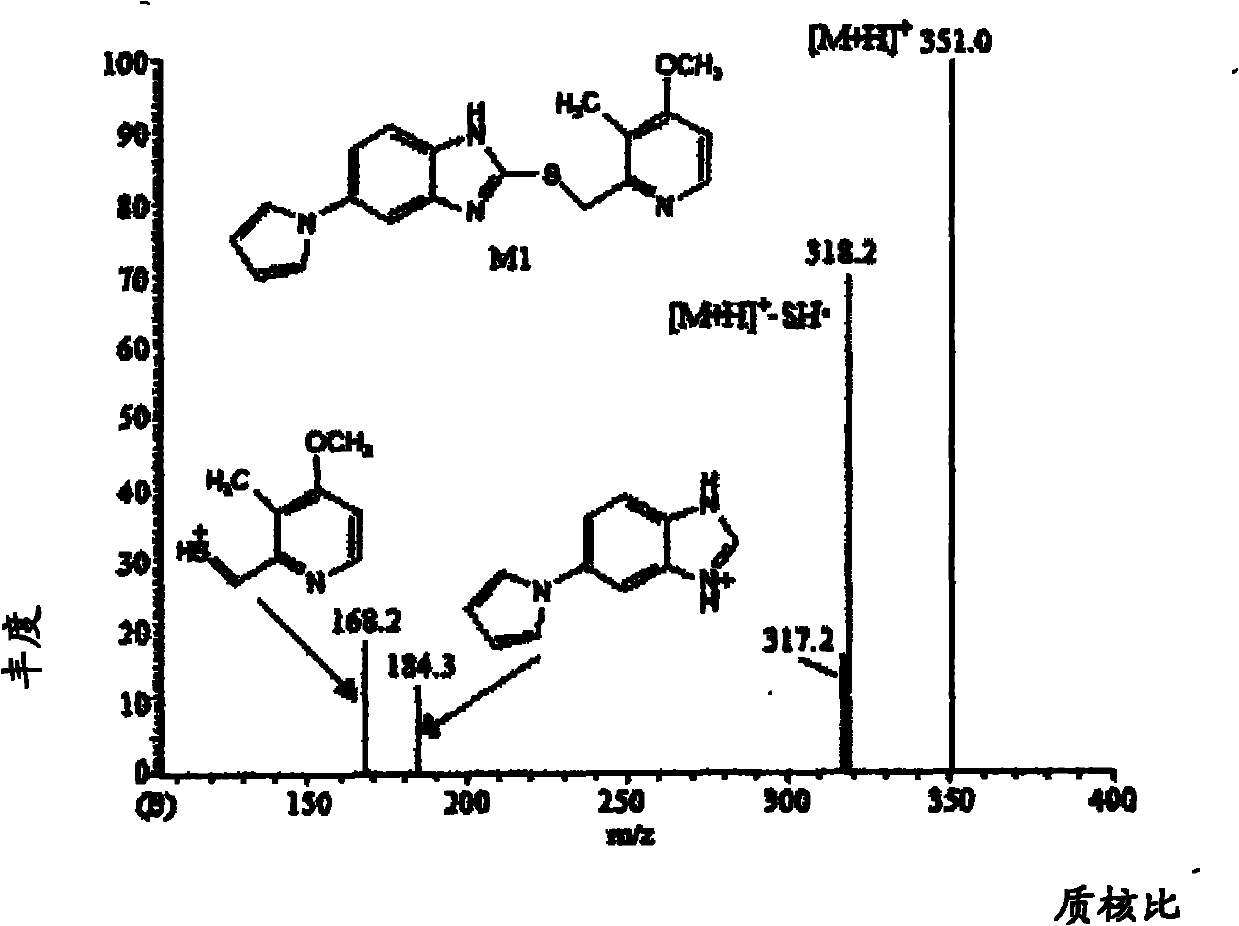
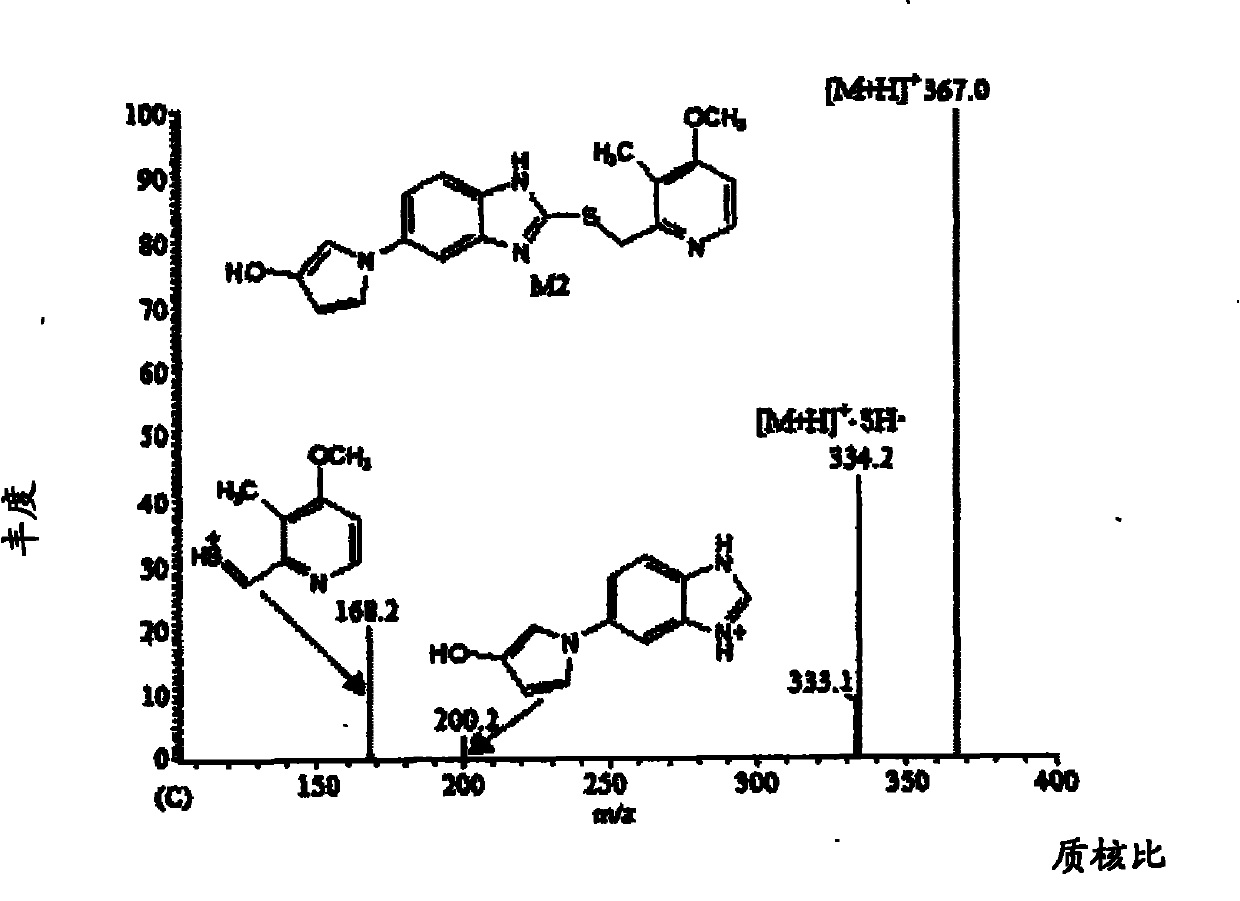
Method for detecting metabolite of ilaprazole in urine
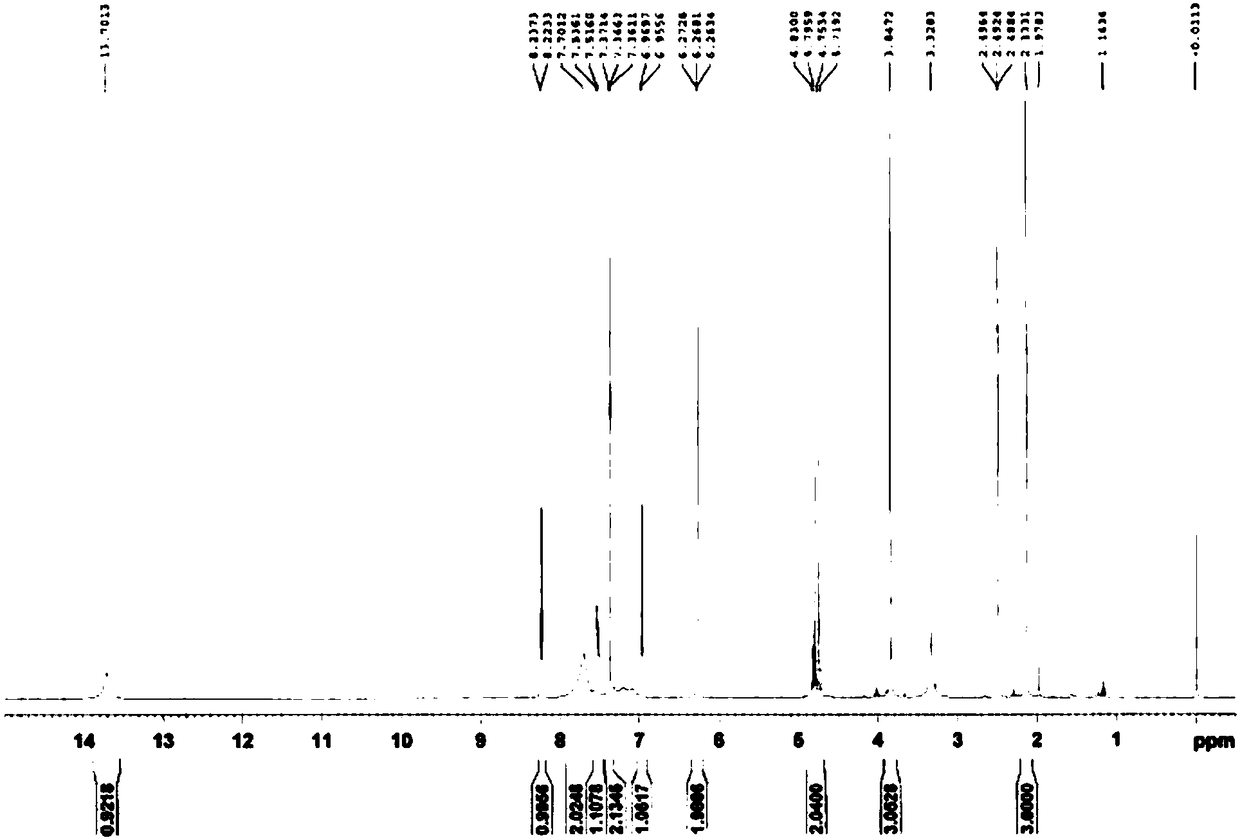
The invention provides a method for detecting metabolite of ilaprazole in urine. The method comprises the following steps of: (1) adding Na2CO3 solution into the urine for precipitation, adding dichloromethane solution containing 0.05 mol / L ammonium salt into supernatant for extraction, and drying a solvent through an organic phase to obtain a sample; (2) performing high performance liquid chromatography-mass spectrometry coupling analysis on the sample; and (3) performing high performance liquid chromatography-nuclear magnetic resonance coupling analysis on the sample. The ilaprazole is a novel proton pump inhibitor for treating peptic ulcer. In the method, a high performance liquid chromatography-mass spectrometry coupling analyzer (HPLC-MS / MS) and a stopped-flow high performance liquidchromatography-nuclear magnetic resonance (HPLC-NMR) test are adopted to clarify the structure of the ilaprazole metabolite in the urine of a human body. Four sulfuration metabolites are identified according to MS / MS and NMR data of the existing standard substances of the ilaprazole and the metabolite of the ilaprazole. A result shows that the method can be widely applied to the quick detection and identification of the metabolite of the ilaprazole.
Owner:LIVZON PHARM GRP INC
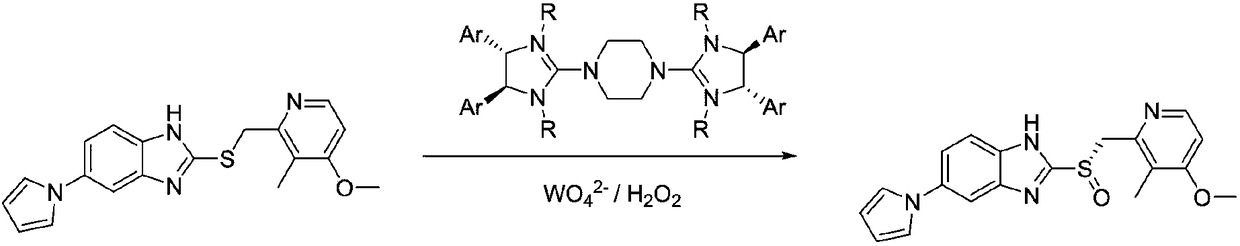
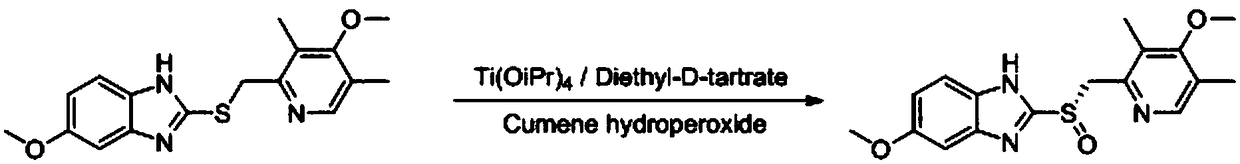
Method for synthesizing chiral ilaprazole
InactiveCN108794451ATroubleshoot compositingNovel processOrganic chemistry methodsSulfite saltDesolvation
Disclosed is a method for synthesizing chiral ilaprazole. The invention belongs to the technical field of pharmacy. At present, reports about ilaprazole chiral compounds are few. The method includes the following steps: under the protection of nitrogen, adding a phase transfer catalyst and isopropyl ether into thioether in order, adding central metal, adding an oxidant, carrying out quenching withan aqueous sodium sulfite solution after reacting at 0 DEG C for 8 hours, carrying out liquid separation, carrying out organic phase desolvation, and then recrystallizing with ethanol to obtain whitecrystals which are the chiral ilaprazole. The method can be used for pharmaceutical research and industrial production of the chiral eprazole. The method has the characteristics of a novel process, few steps, mild reaction conditions and the like.
Owner:SHENZHEN HUAXIAN PHARMA TECH CO LTD
Optically active ilaprazole and synthesis method thereof
The invention relates to optically active ilaprazole and a synthesis method thereof, belongs to the field of pharmaceutical technology, and discloses a method for synthesizing optically active ilaprazole. According to the method, corresponding thiol is used as a starting raw material, and reacts with acrylate or halopropionate to obtain a thioether compound, the thioether compound is oxidized to obtain racemic sulfoxide, and the racemic sulfoxide reacts with another fragment under the action of a chiral phase transfer catalyst to obtain the chiral ilaprazole. According to the present invention, the preparation of the chiral ilaprazole is firstly reported, the synthesis method of the chiral ilaprazole does not require the use of heavy metals and the use of dangerous peroxides, and the excessive oxidation by-product is generated at the penultimate chemical step so as to easily control; and the method has characteristics of novel process, few steps, mild reaction conditions and the like.
Owner:SHENZHEN HUAXIAN PHARMA TECH CO LTD
Preparation method of ilaprazole
ActiveCN103073536BShort reaction timeThe reaction is easy to operateOrganic chemistryChloroformIlaprazole
The invention provides a preparation method of ilaprazole. According to the method, ilaprazole is prepared through a thioether intermediate; obtained ilaprazole is high in quality and has no overoxidation impurities; the method is simple and easy, high in yield, low in cost, environment-friendly, and suitable for industrial production, and has no need for special equipment; and no toxic chloroform solvent is used.
Owner:LIVZON PHARM GRP INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com