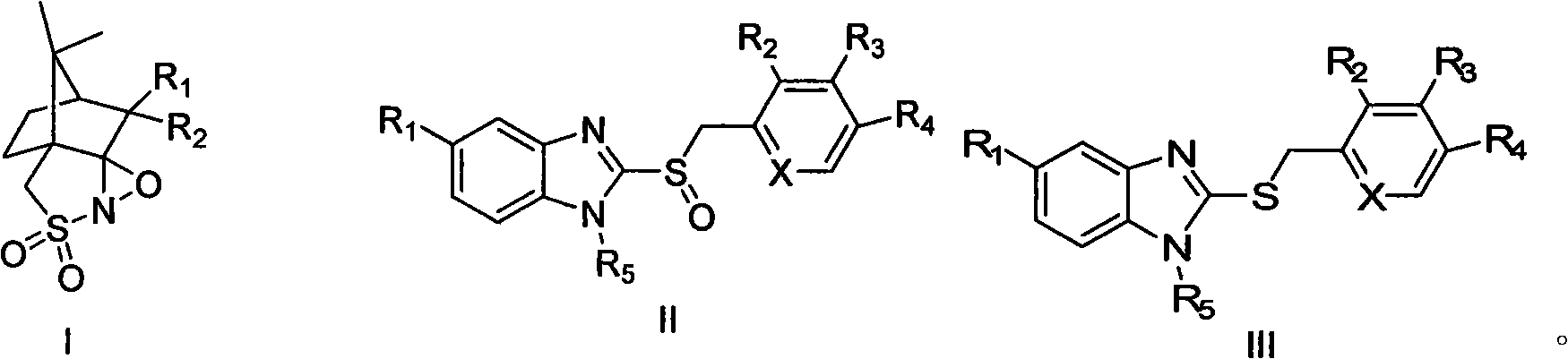
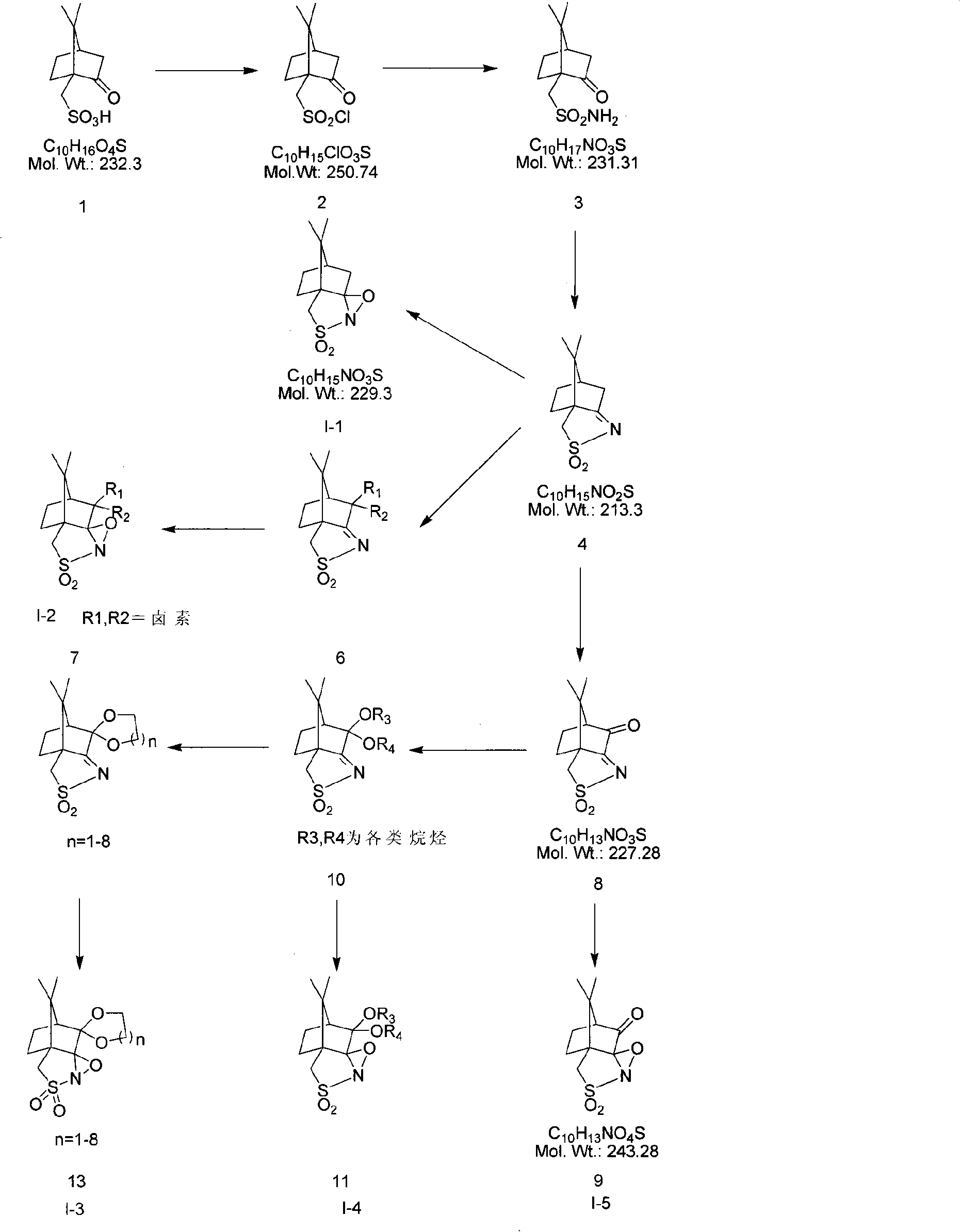
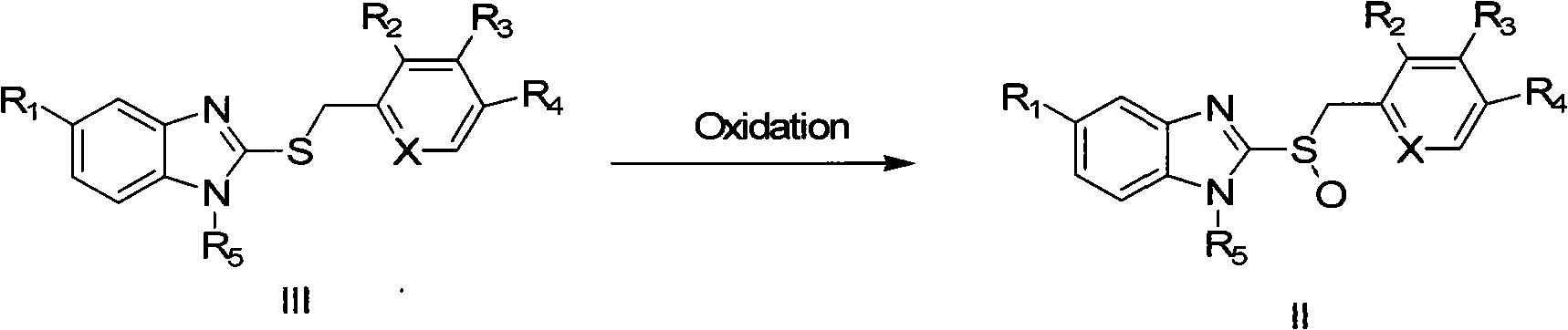
Method for synthesizing high antipode content benzimidazole derivative by unsymmetrical oxidizing thioether into sulphoxide
A technology of benzimidazole and enantiomers, which is applied in the field of enantiomers with high content, to achieve the effect of reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The synthesis of embodiment 1 camphorsulfonamide
[0049] Take a 250ml one-mouth bottle and add 110ml SOCl 2 , add 100g of raw material 1 in portions, stir at room temperature for 10min, the solid is insoluble, install a reflux condenser, heat to reflux for reaction (oil bath T=80°C), the solid gradually dissolves. After dissolving, the reaction liquid was brown, reacted for 1.5h, evaporated SOCl under reduced pressure 2 , to obtain brown solid 2. Add 900ml CH to solid 2 2 Cl 2 , the solid gradually dissolves, and the solution is ready for use. Take a 3L three-neck bottle, add 900ml of ammonia water, cool it to 0°C, stir vigorously, add 900ml2 of CH 2 Cl 2 solution, a large amount of white smoke was generated during the dropwise addition, and the reaction solution gradually changed from a colorless clear liquid to a light yellow turbid liquid, and continued to react at 0°C for 6 hours after the dropwise addition. After the reaction was completed, the solid was fi...
Embodiment 2
[0050] The synthesis of embodiment 2 camphorsulfonylimide
[0051] Take a 1L single-necked bottle, weigh 40g of substance 3 and 34.5g of p-toluenesulfonic acid into the bottle, add 400ml of toluene, heat to reflux for reaction (oil bath T=120°C), the solid gradually dissolves, and continue to heat and reflux for 4h after dissolution. After the reaction was completed, the reaction solution was brown, and 200mlCH was added 2 Cl 2 , a solid precipitated, and the solid was filtered out. The solid was khaki and insoluble with CH 2 Cl 2 , and the filtrate was evaporated to dryness to obtain a brown solid, which was recrystallized from ethanol to obtain 32.48 g of white crystals, with a yield of 88%.
Embodiment 3
[0052] The synthesis of embodiment 3 camphorsulfonyl peroxides
[0053] Method 1: Take a 2L single-mouth bottle, weigh 10g of substance 4 into the bottle, add 300ml of toluene and stir, then add 136gK 2 CO 3 200ml aqueous solution of 87g Oxone was added dropwise to 350ml aqueous solution of 87g Oxone with ice bath to react to 0 ℃, under vigorous stirring, white solid began to form in the reaction solution, and the reaction solution became turbid. After the dropwise addition, the reaction was stirred at room temperature, and the pH of the reaction solution was kept at 9. After 1 day of reaction, 100ml of aqueous solution of 30gOxone was added, and after 3 days of reaction, 100ml of aqueous solution of 30gOxone was added for a total of 5 days of reaction. After the reaction was completed, the solid was filtered out, and the filter cake was washed with CH 2 Cl 2 Fully soak and wash, the filtrate is separated into the organic layer, and the water layer is washed with 200ml×2 C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com