Ilaprazole sodium compound and pharmaceutical composition thereof
A technology of ilaprazole sodium and compounds, applied in the field of pharmaceutical compositions containing said ilaprazole sodium compounds, capable of solving problems such as unmentioned key quality attributes of crystal form stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Embodiment 1: Preparation of B crystal form of ilaprazole sodium
[0080] Method 1: Add 2.5g of ilaprazole sodium raw material drug into 100ml of methanol, stir and dissolve at 25°C, filter to remove insoluble matter, rotate the filtrate at 25°C for crystallization, filter, and dry to obtain ilaprazole sodium type B crystal 2.2 g. Yield 88%.
[0081] Method 2: Add 2.5g of ilaprazole sodium crude drug into 50ml of methanol, stir and dissolve at 50°C, filter to remove insoluble matter, and the filtrate is crystallized by rotary evaporation at 50°C, filter, and dry to obtain ilaprazole sodium type B crystal 2.24 g. Yield 89.6%.
[0082] Method 3: Add 2.5g of ilaprazole sodium raw material drug into 100ml of ethanol, stir and dissolve at 50°C, filter to remove insoluble matter, rotate the filtrate at 50°C for crystallization, filter, and dry to obtain ilaprazole sodium type B crystal 2.3 g. Yield 92%.
[0083] Method 4: Add 2.5g of ilaprazole sodium crude drug into a ...
Embodiment 2
[0088] Embodiment 2: Preparation of C crystal form of ilaprazole sodium
[0089] Method 1: Suspend 2.5 g of ilaprazole sodium crude drug in 100 ml of ethyl acetate, stir at 25°C for 24 hours, filter, and dry to obtain 2.22 g of ilaprazole sodium type C crystals. Yield 88.8%.
[0090] Method 2: Suspend 2.5 g of ilaprazole sodium crude drug in 100 ml of acetonitrile, stir at 25°C for 24 hours, filter, and dry to obtain 2.28 g of ilaprazole sodium type C crystals. Yield 91.2%.
[0091] Method 3: Suspend 2.5 g of ilaprazole sodium crude drug in 50 ml of ethyl acetate, stir at 50° C. for 24 hours, filter, and dry to obtain 2.26 g of ilaprazole sodium type C crystals. Yield 90.4%.
[0092] Method 4: Suspend 2.5 g of ilaprazole sodium crude drug in 60 ml of acetonitrile, stir at 50° C. for 24 hours, filter, and dry to obtain 2.3 g of ilaprazole sodium type C crystals. Yield 92%.
[0093] Method 5: Add 2.5g of ilaprazole sodium crude drug into a beaker filled with 30ml of isoprop...
Embodiment 3
[0103] Embodiment 3: Preparation of D crystal form of ilaprazole sodium
[0104] Method 1: Suspend 2.5 g of ilaprazole sodium crude drug in 100 ml of chloroform, stir at 25°C for 24 hours, filter, and dry to obtain 2.45 g of ilaprazole sodium type D crystals. Yield 98%.
[0105] Method 2: Suspend 2.5 g of ilaprazole sodium crude drug in 50 ml of chloroform, stir at 50° C. for 24 hours, filter, and dry to obtain 2.25 g of ilaprazole sodium type D crystals. Yield 90%.
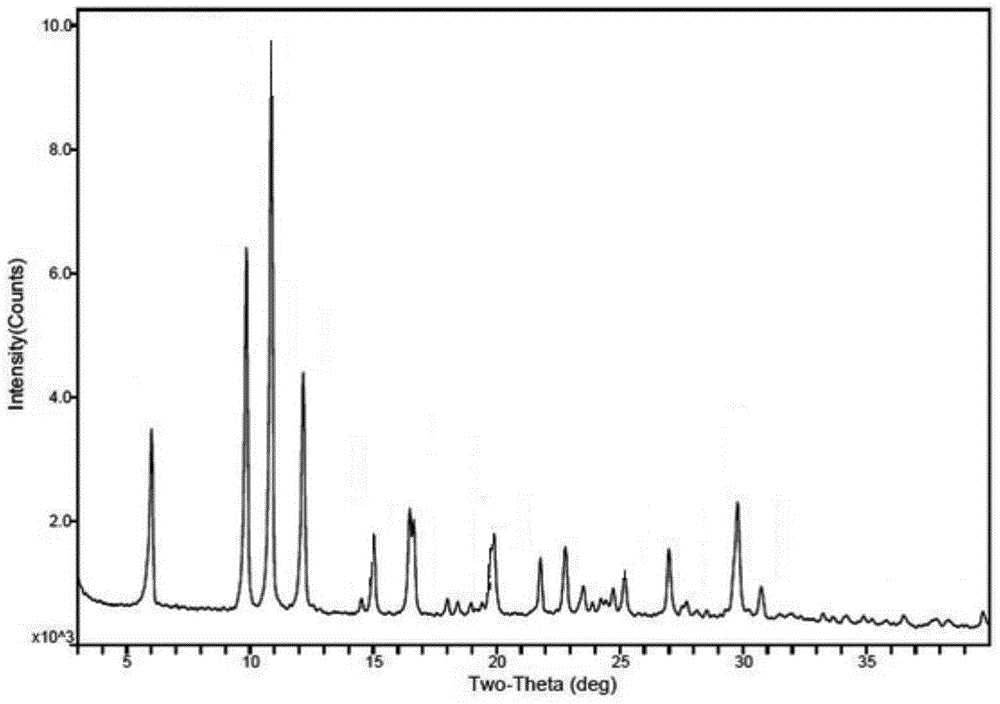
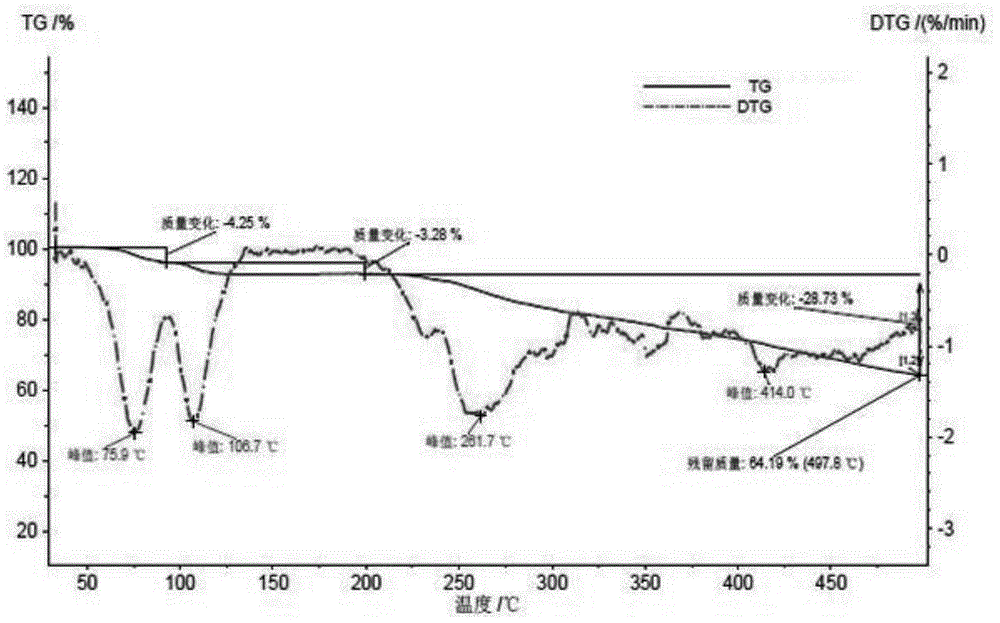
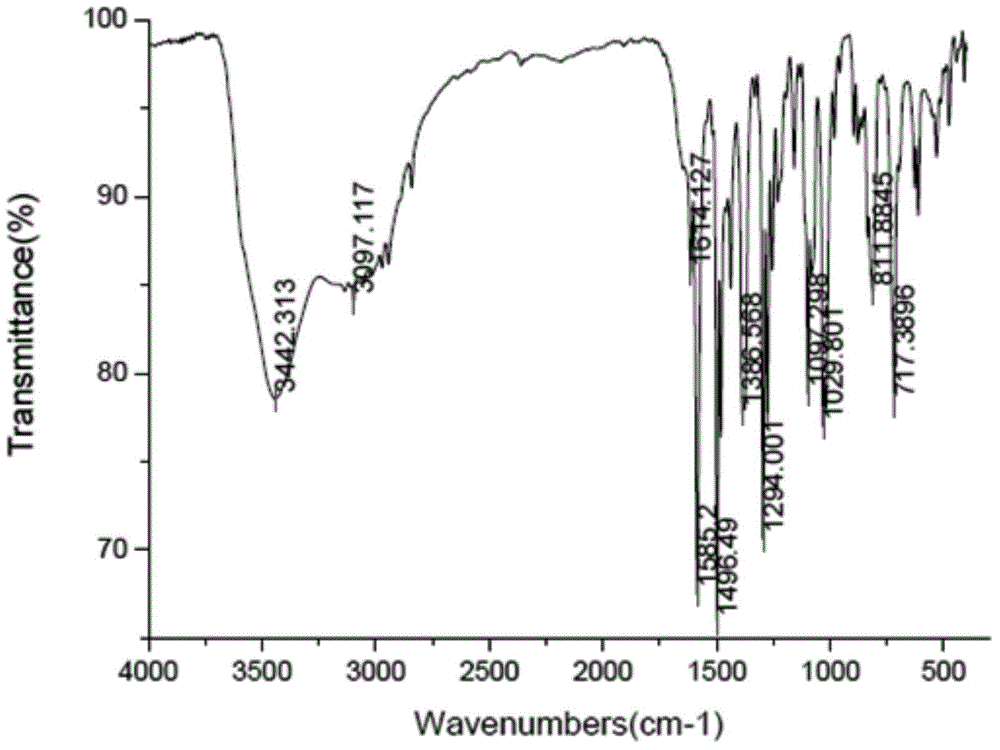
[0106] Polarized photos show that the above-mentioned ilaprazole sodium compound D-type crystals are powdery crystals, and the XRPD spectrum measured by Cu-Kα radiation is basically as follows: Figure 13 As shown, the spectral data are listed in Table 4 below. The TGA spectrum is basically as Figure 14 As shown, the crystal form has a weight loss of about 17.2% at 30-200°C, and the corresponding DSC has an endothermic peak at about 74.7°C. The IR spectrum is basically as Figure 15 shown. The Raman spect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com