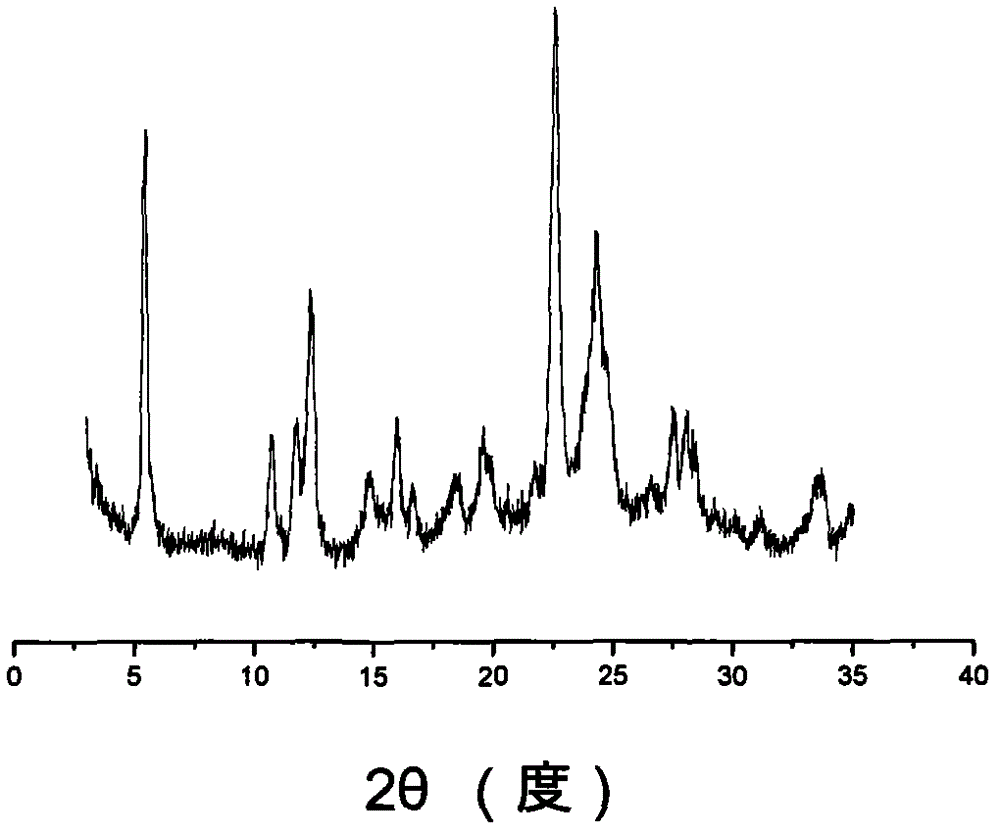
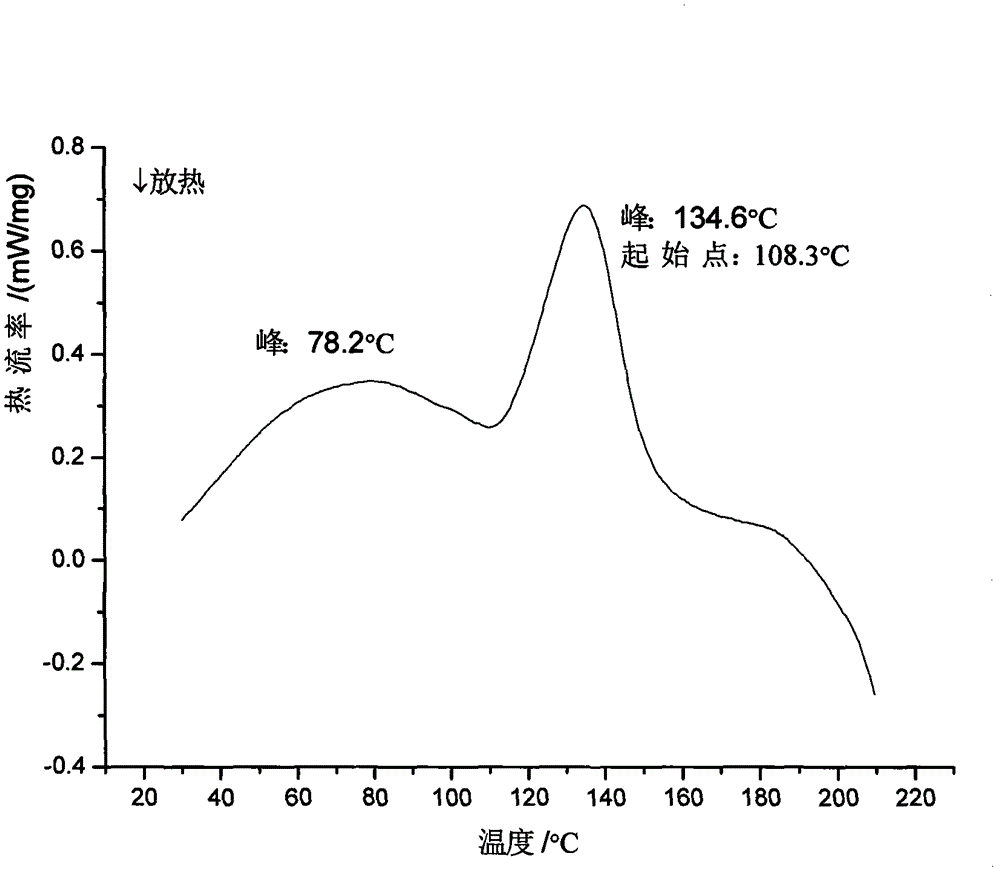
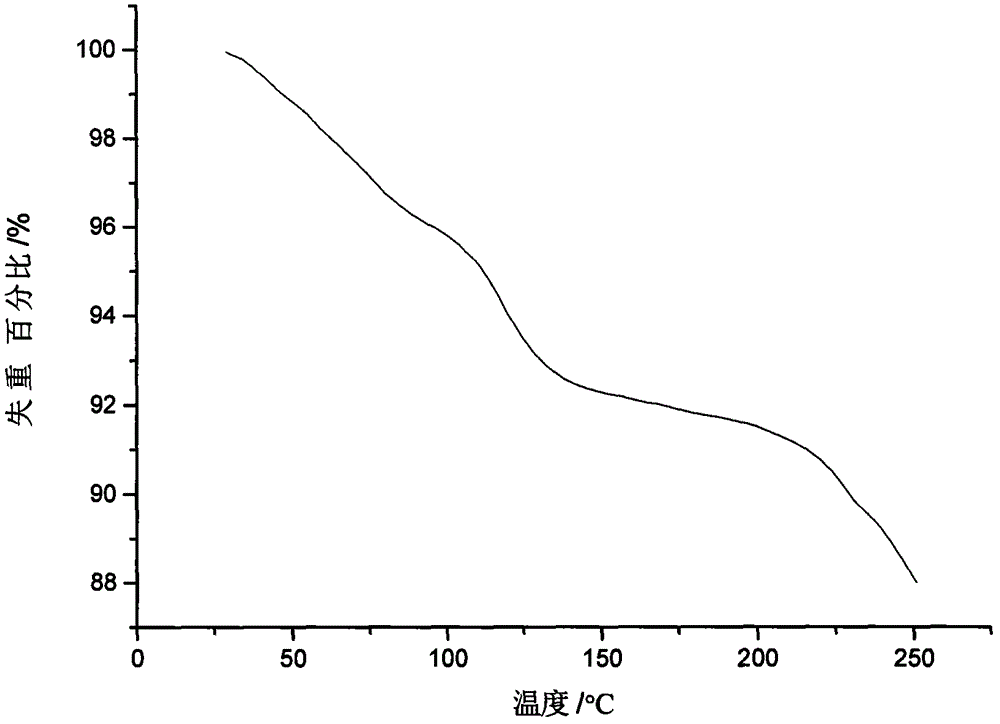
Ilaprazole sodium crystal form and preparation method thereof
A technology of ilaprazole sodium and its crystal form, which is applied in the field of ilaprazole sodium new crystal form and its preparation, and can solve the problems of no ilaprazole sodium crystal form, limited clinical application, gastric acid secretion rebound, etc. problems, to achieve the effect of easy preparation, low preparation cost, and less amount of solvent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] at 25°C with CH 2 Cl 2 : a mixed solution of methanol=10:1 (volume ratio), 5.5 mL of which was added dropwise with 1 drop of triethylamine, 300 mg of ilaprazole sodium was added to the solution, stirred to dissolve, filtered, and the filtrate was placed in a fume hood to volatilize. After a few hours, the solvent evaporated completely and dried under vacuum. 282 mg of pale yellow crystalline powder were obtained with a yield of 94%.
Embodiment 2
[0036] at 25°C with CH 2 Cl 2 : methanol=10:1 (volume ratio) mixed solution, 1 mL of which was added dropwise with 1 drop of triethylamine, 60 mg of ilaprazole sodium was added to the solution, stirred and dissolved, filtered, and the filtrate was placed in a fume hood to volatilize, and several After 1 hour, the solvent evaporated completely, and vacuum-dried. 55 mg of pale yellow crystalline powder were obtained, with a yield of 91.7%.
Embodiment 3
[0038] at 27°C with CH 2 Cl 2 : a mixed solution of methanol=10:1 (volume ratio), 5.5 mL of which was added dropwise with 1 drop of triethylamine, 300 mg of ilaprazole sodium was added to the solution, stirred to dissolve, filtered, and the filtrate was placed in a fume hood to volatilize. After a few hours, the solvent evaporated completely and dried under vacuum. 272 mg of pale yellow crystalline powder were obtained with a yield of 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com