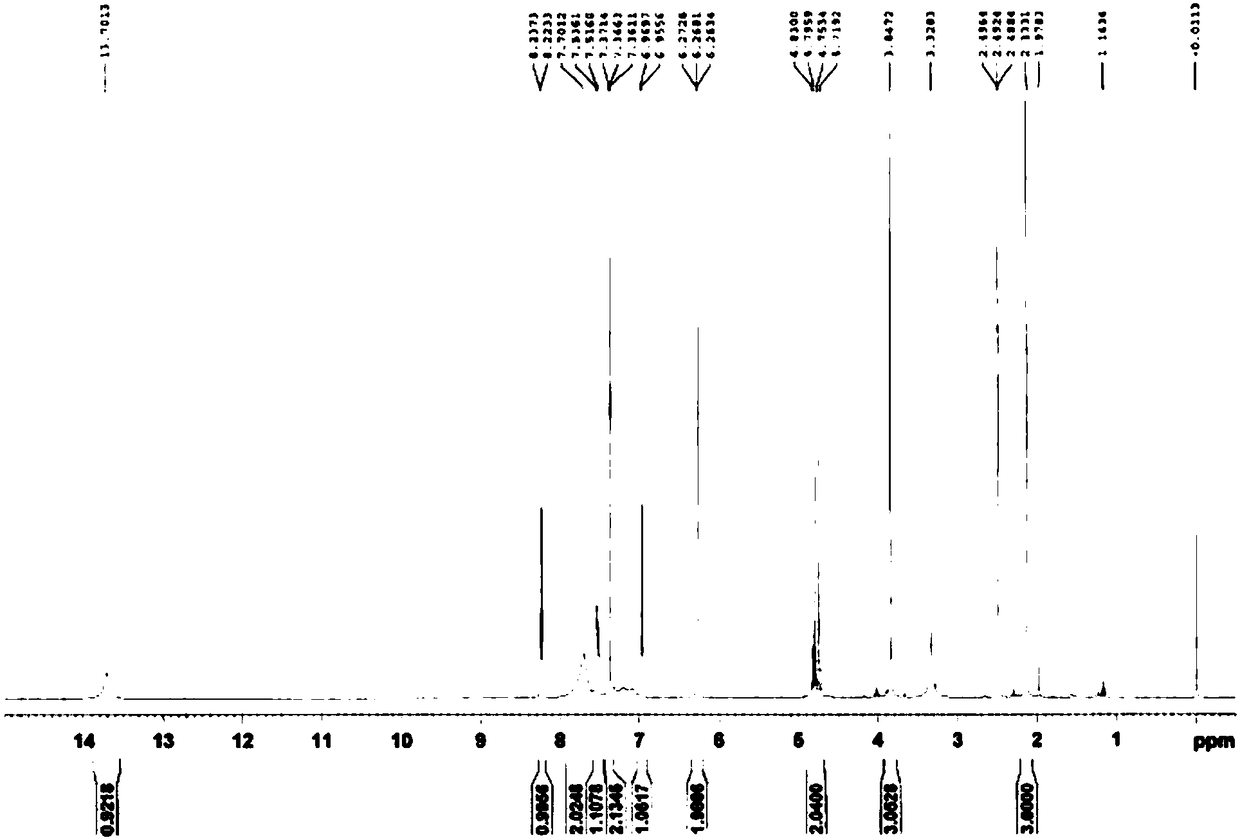
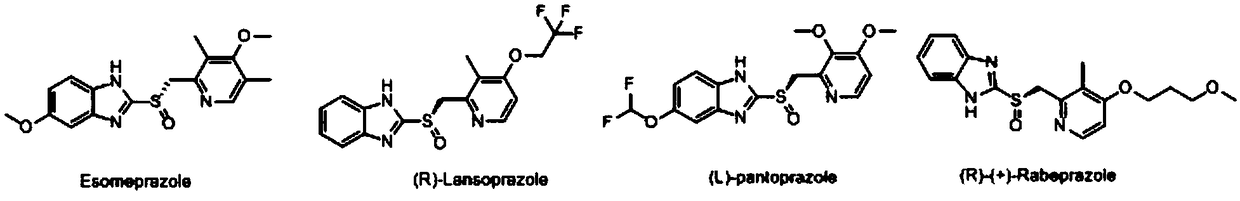
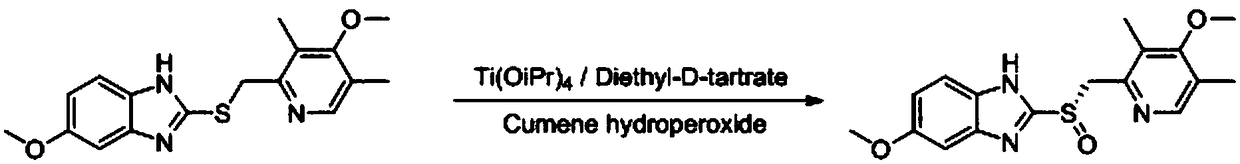
Optically active ilaprazole and synthesis method thereof
A technology of ilaprazole and optical activity, applied in the field of optically active ilaprazole and its new process route, can solve the problems of ilaprazole not easy to split, many by-products in the oxidation step, and difficult to control metal residues, etc. Achieve the effect of novel process, mild reaction conditions and few steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. Synthesis of Thioether Compounds
[0031] Take a 100mL three-necked flask, add 5-(1H-pyrrol-1-yl)-1H-benzimidazole-2-thiol (2.15g, 0.01mol), ethanol (10mL) and Triethylamine (0.1 g) was then carefully added ethyl acrylate (1.10 g, 0.011 mol). After the reaction is completed, the solvent is removed, and the product is directly used in the next step.
[0032] 2. Synthesis of racemic sulfoxide compounds
[0033] Take a 100mL three-necked flask, and add the product from the previous step, acetonitrile (20mL) and 10% aqueous sodium hydroxide solution (5mL) to it under nitrogen protection, and then carefully add 10% aqueous sodium hypochlorite (8.2mL, 1.1eq. ). After the reaction was completed, it was acidified to pH=3 with dilute hydrochloric acid, and filtered. The filter cake is beaten with ethanol, filtered, and put into the next step after vacuum drying.
[0034] 3. Synthesis of Chiral Ilaprazole
[0035] Get a 100mL three-necked flask, add the product of the pr...
Embodiment 2
[0041] 1. Synthesis of Thioether Compounds
[0042] Take a 100mL three-necked flask, add 5-(1H-pyrrol-1-yl)-1H-benzimidazole-2-thiol (2.15g, 0.01mol), ethanol (10mL) and Triethylamine (1.1 g) was then carefully added ethyl bromopropionate (1.80 g, 0.011 mol). After the reaction is completed, filter and remove the solvent, and the product is directly used in the next step.
[0043] 2. Synthesis of racemic sulfoxide compounds
[0044] Take a 100mL three-necked flask, under the protection of nitrogen, add the product from the previous step, acetonitrile (20mL), and then carefully add m-chloroperoxybenzoic acid (1.9g, 1.1eq.). After the reaction is complete, filter. The filtrate was concentrated, 10 ml of methanol was added, filtered, and the filter cake was vacuum-dried and used in the next step.
[0045] 3. Synthesis of Chiral Ilaprazole
[0046] Get a 100mL three-necked flask, add the product of the previous step, methylcyclohexane (20mL) and 67% cesium hydroxide solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com