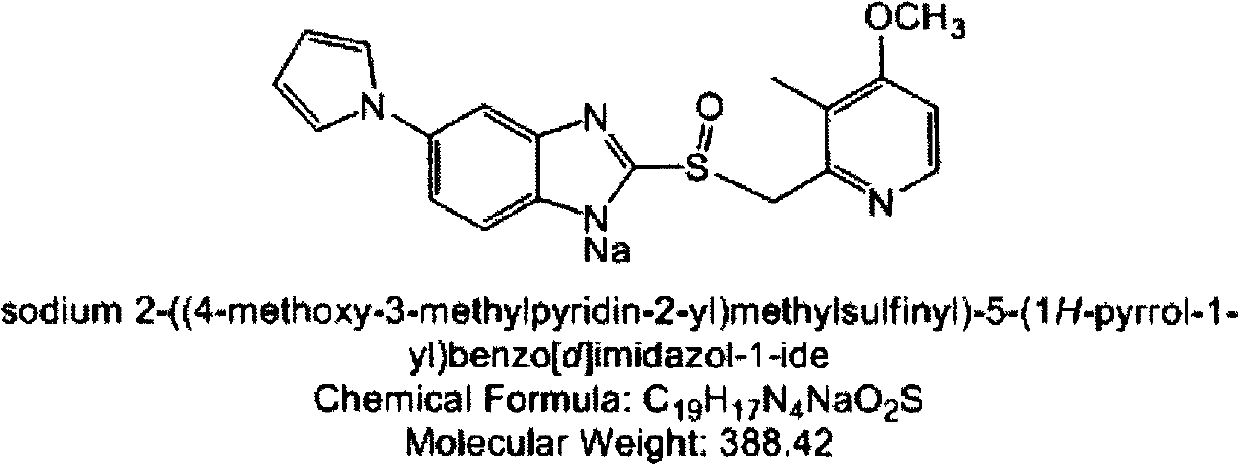
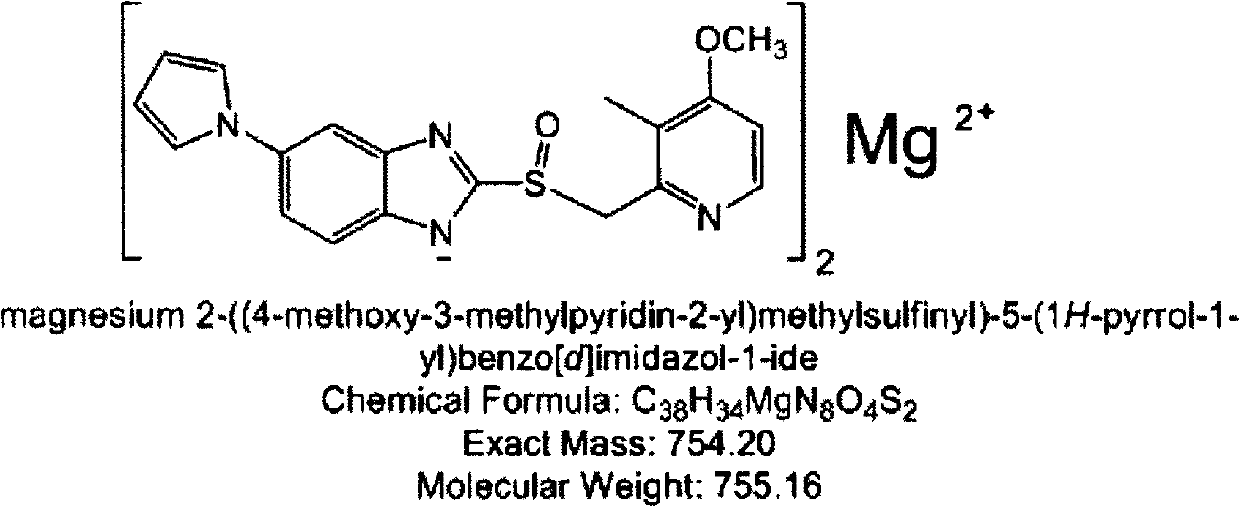
Ilaprazole enteric capsule and preparation method thereof
A technology of enteric-coated capsules and ilaprazole, which is applied in the field of salt-coated enteric-coated capsules and its preparation, can solve problems such as large fluctuations in enteric-coated properties of blank capsule shells, affecting product quality stability of enteric-coated products, etc., and achieve effective It is beneficial to human health and safety, improves the bioavailability of drugs, and has the effect of good acid resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Preparation of pellets coated with ilaprazole sodium drug-loaded layer by centrifugal granulation
[0037] Table 1 Prescription of micropills containing ilaprazole sodium
[0038] material
amount (g)
Blank pellet core (0.2-0.3mm)
400
29
29
Polyvinylpyrrolidone (PVP K30)
50
water
[0039] Preparation of 5% (m / m) polyvinylpyrrolidone aqueous solution: weigh 50 g of PVP and dissolve in 25% (v / v) ethanol aqueous solution. Weigh 400 g of 5% (m / m) PVP solution, add the prescribed amount of sodium hydroxide, adjust the pH to about 12, then weigh ilaprazole sodium and dissolve it therein. Weigh 500 g of blank pellet cores (sucrose pellet cores) and pour them into the feed pot, turn on the host, adjust the host speed, air volume, jet pressure, and spray rate, and spray the prepared drug-containing solution. After spraying the liquid medicine, continue...
Embodiment 2
[0042] Example 2: Preparation of pellets coated with ilaprazole magnesium drug-loaded layer by centrifugal granulation
[0043] Table 3 Prescription of pellets containing ilaprazole magnesium
[0044] material
[0045]Processing of raw materials and auxiliary materials: sieve magnesia and mannitol through 100 meshes, weigh the prescribed amount of micropowder silica gel and mix them evenly with magnesia, mannitol and CMS-Na for later use. Preparation of adhesive: preparation of 5% PVP solution: weigh 50 g of PVP and dissolve in 95% (v / v) ethanol aqueous solution. Weigh the prescribed amount of ilaprazole sodium and sodium hydroxide into a certain amount of 5% PVP solution and stir to dissolve. Weigh the prescribed amount of blank pellet cores (microcrystalline cellulose pellet cores) and pour them into the pot, turn on the host, adjust the host speed, air volume, jet pressure, and liquid spray rate, and spray the prepared drug-containing solution. Pour the mixed ex...
Embodiment 3
[0046] Example 3: Preparation of pellets coated with ilaprazole lithium drug-loaded layer by centrifugal granulation
[0047] Table 4 Prescription of micropellets containing ilaprazole lithium
[0048] material
[0049] Processing of raw materials and auxiliary materials: Magnesium hydroxide and pregelatinized starch are sieved through 100 meshes, and the prescribed amount of talcum powder is weighed and mixed with magnesium hydroxide, pregelatinized starch and low-substituted cellulose sodium for later use. Preparation of adhesive: preparation of 5% HPMC solution: weigh 50 g of HPMC and dissolve in 95% (v / v) ethanol aqueous solution. Weigh the prescribed amount of ilaprazole lithium and magnesium hydroxide and add to a certain amount of 5% HPMC solution and stir to dissolve. Weigh the prescribed amount of blank pellet cores (microcrystalline cellulose pellet cores) and pour them into the pot, turn on the host, adjust the host speed, air volume, jet pressure, and li...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com