Method for preparing hyperbranched polyester modified organic silicon resin
A technology of polyester modification and silicone, which is applied in the direction of coating, etc., can solve the problems of unsatisfactory heat resistance, acid resistance and salt spray resistance, etc., and achieve excellent salt spray resistance, water and solvent resistance, and high The effect of heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
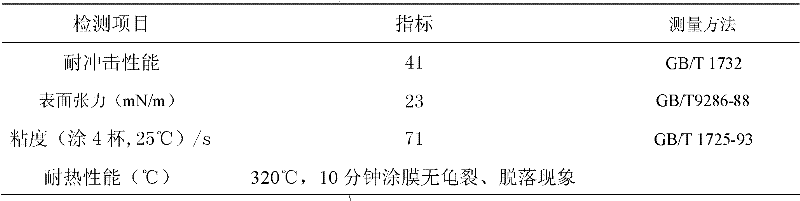
Embodiment 1
[0040] 1) Hydroxy-terminated hyperbranched polyester
[0041] a) Composition:
[0042] Trihydric alcohol: 96g (0.8mol) of trimethylolethane; 25.2g (0.2mol) of 1,3,5-benzenetriol;
[0043] Dibasic acid: 132.8g (0.8mol) of terephthalic acid; 23.6g (0.2mol) of succinic acid;
[0044] b) synthesis steps:
[0045] Add the trihydric alcohol and the dibasic acid into a reactor equipped with heating, condensation and nitrogen protection, raise the temperature to 110°C under nitrogen protection, and keep it for 2h; heat it to 240°C at a heating rate of 20°C per hour, and keep it at 1.33kPa for 4h ; At last, room temperature was cooled to obtain 235g terminal hydroxyl hyperbranched polyester.
[0046] c) Properties of hydroxyl-terminated hyperbranched polyesters
[0047] The weight-average molecular weight of the hydroxyl-terminated hyperbranched polyester was detected by gel permeation chromatography, and the result was 3998.
[0048] The hydroxyl value was tested according to the...
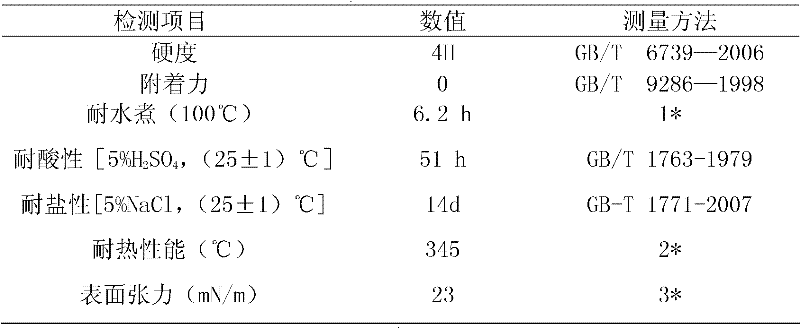
Embodiment 2
[0075] 1) Hydroxy-terminated hyperbranched polyester
[0076] a) Composition:
[0077] Trihydric alcohol: 168g (1.4mol) of trimethylolethane; 37.8g (0.3mol) of 1,2,4-benzenetriol; 37.8g (0.3mol) of 1,3,5-benzenetriol;
[0078] Dibasic acid: 66.4g (0.4mol) of isophthalic acid; 66.4g (0.4mol) of terephthalic acid; 29.2g (0.2mol) of adipic acid;
[0079] b) synthesis steps:
[0080] Add tribasic alcohol and dibasic acid into a reactor equipped with heating, condensation and nitrogen protection, raise the temperature to 110°C under nitrogen protection, and keep it for 2h; heat it to 240°C at a heating rate of 20°C per hour, and keep it for 2h at 0.66kPa ; Finally, room temperature was cooled to obtain 364g terminal hydroxyl hyperbranched polyester.
[0081] c) Properties of hydroxyl-terminated hyperbranched polyesters
[0082] The weight-average molecular weight of the hydroxyl-terminated hyperbranched polyester is detected by gel permeation chromatography, and the result is 1...
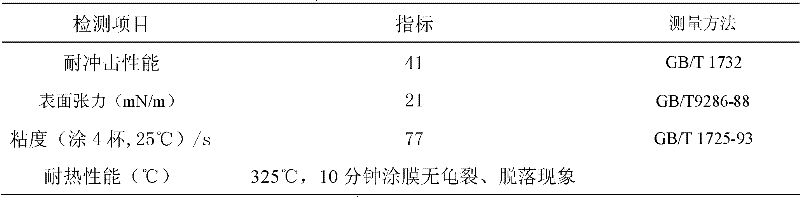
Embodiment 3
[0105] 1) Hydroxy-terminated hyperbranched polyester
[0106] a) Composition:
[0107] Triol: Trimethylolethane 192g (1.6mol);
[0108] Dibasic acid: 157.7g (0.95mol) of isophthalic acid; 7.3g (0.05mol) of adipic acid;
[0109] b) synthesis steps:
[0110] Add tribasic alcohol and dibasic acid into a reactor equipped with heating, condensation and nitrogen protection, raise the temperature to 110°C under nitrogen protection, and keep it for 2 hours; heat it to 240°C at a heating rate of 20°C per hour, and keep it at 0.78kPa for 2.5 h; finally cooled to room temperature to obtain 310g hydroxyl-terminated hyperbranched polyester.
[0111] c) Properties of hydroxyl-terminated hyperbranched polyesters
[0112] The weight-average molecular weight of the hydroxyl-terminated hyperbranched polyester was detected by gel permeation chromatography, and the result was 2611.
[0113] The hydroxyl value was tested according to the method described in GB / T7193.2-1987, and the result was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydroxyl value | aaaaa | aaaaa |
| Hydroxyl value | aaaaa | aaaaa |
| Hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com