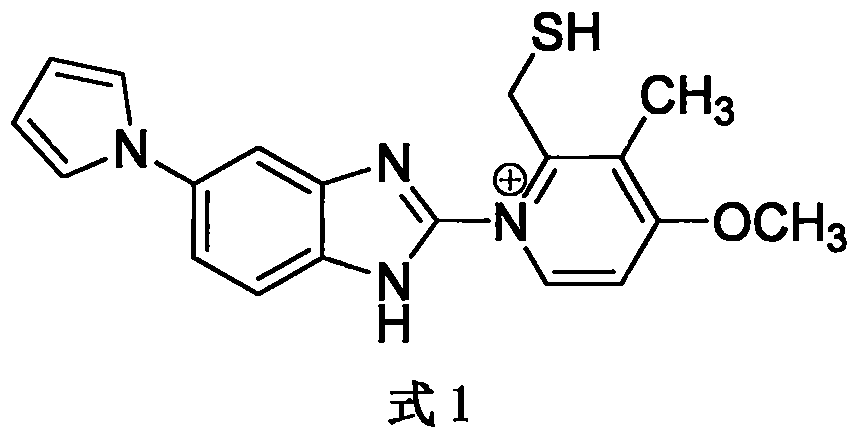
Sodium ilaprazole freeze-dried powder injection
A technology of ilaprazole sodium and freeze-dried powder injection, which is applied in the field of freeze-dried powder injection of proton pump inhibitor sodium salt, which can solve the problems of venous blood vessel injury, granulation tissue, hyperplasia, and irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
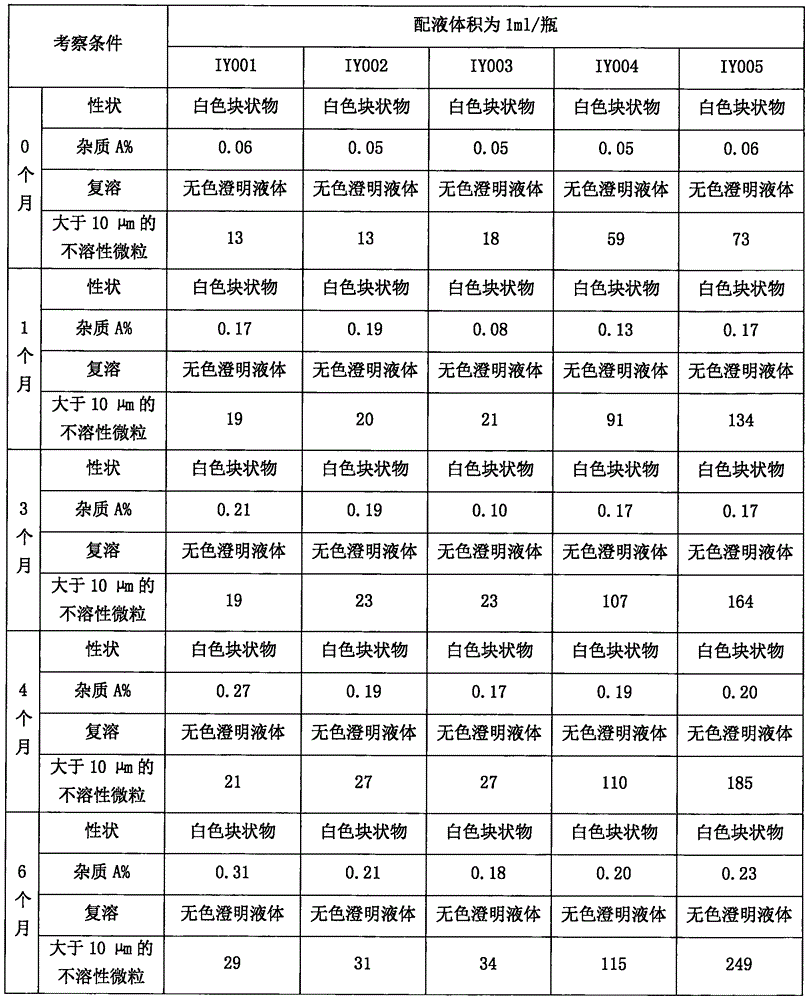
[0040] This embodiment is to adopt the method of the present invention to prepare 1000 bottles of ilaprazole sodium freeze-dried powder injection (marked amount: containing 10mg ilaprazole sodium, 0.2% edetate disodium), the specific process includes the following steps
[0041] 1) Pre-cool 900ml of water for injection to below 25°C.
[0042] 2) First weigh about 800ml of water for injection, add 2.0g of edetate disodium, and then adjust the pH to 11.5-12.0 with 5mol / L sodium hydroxide. Add 10g of ilaprazole sodium crude drug, fully dissolve, add water for injection to 1000g, and then adjust the pH to 11.5-12.0 with 5mol / L sodium hydroxide. Close the lid of the ingredient jar after dissolving.
[0043] 3) The solution is filtered through two 0.2um filter membranes to the batching room.
[0044] 4) Use an automatic filling machine (Nanjing Bojian Technology Co., Ltd. KFG~300 linear semi-dropper aseptic filling machine) to pack the filtrate into 1000 bottles (specification: 10...
Embodiment 2
[0047] This embodiment is to adopt the method of the present invention to prepare 1000 bottles of ilaprazole sodium freeze-dried powder injection (marked amount: containing 10mg ilaprazole sodium, 0.15% edetate disodium), the specific process includes the following steps
[0048] 1) Pre-cool 900ml of water for injection to below 25°C.
[0049] 2) First weigh about 800ml of water for injection, add 1.5g of edetate disodium, and then adjust the pH to 11.5-12.0 with 5mol / L sodium hydroxide. Add 10g of ilaprazole sodium crude drug, fully dissolve, add water for injection to 1000g, and then adjust the pH to 11.5-12.0 with 5mol / L sodium hydroxide. Close the lid of the ingredient jar after dissolving.
[0050] 3) The solution is filtered through two 0.2um filter membranes to the batching room.
[0051] 4) Use an automatic filling machine (Nanjing Bojian Technology Co., Ltd. KFG~300 linear semi-dropper aseptic filling machine) to pack the filtrate into 1000 bottles (specification: 1...
Embodiment 3
[0054] This embodiment is to adopt the method of the present invention to prepare 1000 bottles of ilaprazole sodium freeze-dried powder injection (marked amount: containing 10mg ilaprazole sodium, 0.10% edetate disodium), the specific process includes the following steps
[0055] 1) Pre-cool 900ml of water for injection to below 25°C.
[0056] 2) First weigh about 800ml of water for injection, add 1.0g of edetate disodium, and then adjust the pH to 11.5-12.0 with 5mol / L sodium hydroxide. Add 10g of ilaprazole sodium crude drug, fully dissolve, add water for injection to 1000g, and then adjust the pH to 11.5-12.0 with 5mol / L sodium hydroxide. Close the lid of the ingredient jar after dissolving.
[0057] 3) The solution is filtered through two 0.2um filter membranes to the batching room.
[0058] 4) Use an automatic filling machine (Nanjing Bojian Technology Co., Ltd. KFG~300 linear semi-dropper aseptic filling machine) to pack the filtrate into 1000 bottles (specification: 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com