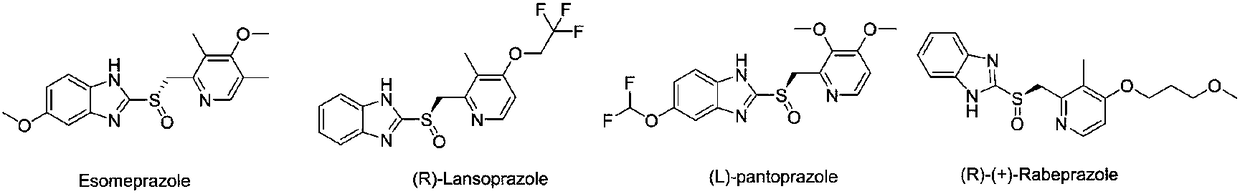
Method for synthesizing chiral ilaprazole
A synthetic method and chiral technology, which is applied in the field of new process route of optically active ilaprazole, can solve the problem that ilaprazole has no chiral compound, and achieve the effect of novel process, fewer steps and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
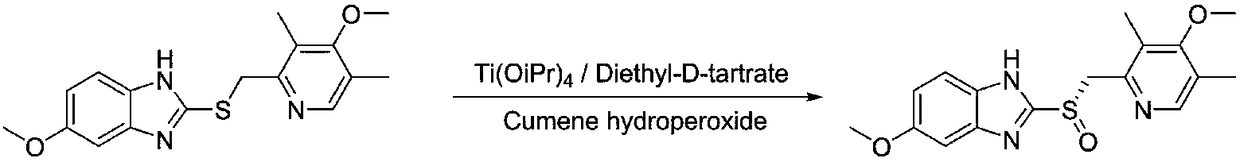
[0022] 1. Synthesis of chiral ilaprazole
[0023] Take a 100 mL three-necked flask and add sulfide (10 mmol), biguanide salt phase transfer catalyst A (0.1 mmol) and isopropyl ether (40 mL) to it in sequence under the protection of nitrogen. Then sodium tungstate (0.2 mmol) was added, followed by aqueous peroxyacetic acid (12 mmol). After reacting at 0°C for 8 hours, it was quenched with sodium sulfite aqueous solution and separated. The organic phase is desolventized, and then recrystallized with ethanol to obtain white crystals with a total yield of 90%, an ee value of 99%, and levorotatory.
[0024] The structure and product structure of catalyst A are as follows:
[0025]
Embodiment 2
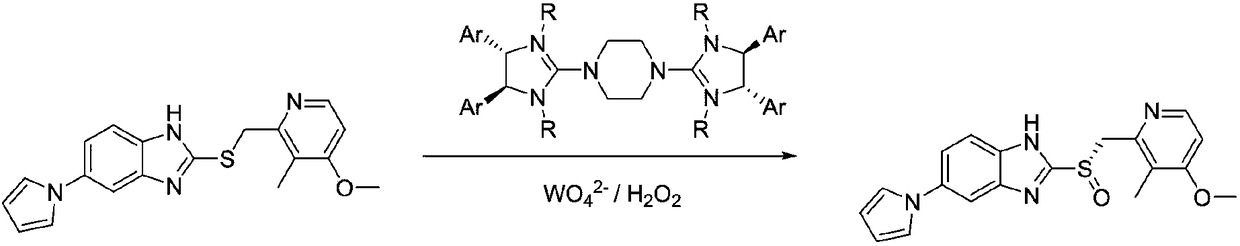
[0027] 1. Synthesis of chiral ilaprazole
[0028] Take a 100 mL three-necked flask and add sulfide (10 mmol), biguanide salt phase transfer catalyst B (0.1 mmol) and isopropyl ether (40 mL) to it in sequence under the protection of nitrogen. Then sodium molybdate (0.2 mmol) was added, followed by aqueous hydrogen peroxide solution (12 mmol). After reacting at 0°C for 8 hours, it was quenched with sodium sulfite aqueous solution and separated. The organic phase was desolventized, and then recrystallized with ethanol to obtain white crystals with a total yield of 90%, an ee value of 99%, and dextrorotation.
[0029] The structure of catalyst A is as follows:
[0030]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com