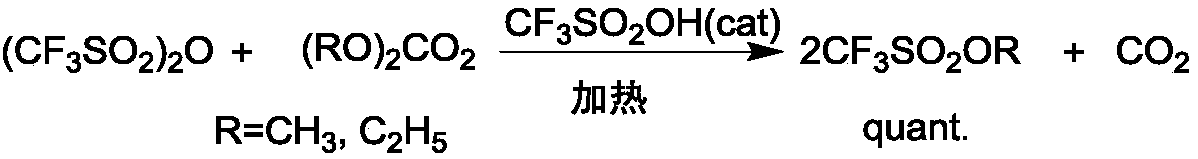
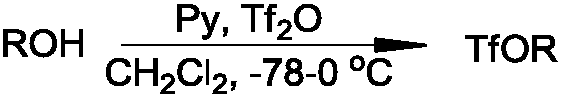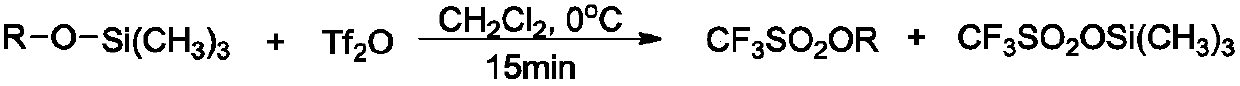The synthetic method of triflate
A technology of trifluoromethanesulfonate and trifluoromethanesulfonic anhydride, which is applied in the field of trifluoromethanesulfonate synthesis, can solve the problems of trifluoromethanesulfonic acid with poor stability, and achieve fast reaction speed and high raw material Inexpensive and readily available raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
[0042] Synthetic one of embodiment 1 methyl trifluoromethanesulfonate (TfOMe)
[0043] Tf 2 O+HC(OMe) 3 →TfoMe+HCO 2 Me
[0044] Under ice bath, trimethyl orthoformate (3.18g, 30mmol) was slowly added to trifluoromethanesulfonic anhydride (8.47g, 30mmol), transferred to 25°C for reaction, nuclear magnetic monitoring for 15 minutes, after the reaction was complete, depressurized Distillation gave 9.74 g of methyl trifluoromethanesulfonate as a colorless liquid, with a yield of 99%.
[0045] TfOMe: b.p.47℃ / 42mmHg; 1 H NMR (400MHz, CDCl 3 )δ4.21(s,3H); 13 C NMR (101MHz, CDCl 3 )δ118.7(q, J=321.2Hz), 61.6; 19 F NMR (376MHz, CDCl 3 )δ-74.5(s,3F).
Embodiment 2 3
[0046] Synthesis of embodiment 2 methyl trifluoromethanesulfonate (TfOMe)
[0047] Tf 2 O+MeC(OMe) 3 →TfoMe+MeCO 2 Me
[0048] Under ice bath, trimethyl orthoacetate (3.60g, 30mmol) was slowly added to trifluoromethanesulfonic anhydride (8.47g, 30mmol), transferred to 25°C for reaction, nuclear magnetic monitoring for 15 minutes, after the reaction was complete, depressurized Distillation gave 9.65 g of methyl trifluoromethanesulfonate as a colorless liquid, with a yield of 98%.
Embodiment 3 3
[0049] The synthesis of embodiment 3 ethyl trifluoromethanesulfonate (TfOEt)
[0050] Tf 2 O+HC(OEt) 3 →TfOEt+HCO 2 Et
[0051]Under ice bath, triethyl orthoformate (4.44g, 30mmol) was slowly added to trifluoromethanesulfonic anhydride (8.47g, 30mmol), transferred to 25°C for reaction, 15 minutes of NMR monitoring, the reaction was completed, and the pressure was reduced Distillation gave 10.15 g of ethyl triflate as a colorless liquid, with a yield of 94%.
[0052] TfOEt: b.p.42°C (42mmHg); 1 H NMR (400MHz, CDCl 3 )δ4.62(q, J=7.1Hz, 2H), 1.51(t, J=7.1Hz, 3H); 13 C NMR (101MHz, CDCl 3 )δ118.6 (q, J=320.8Hz), 74.0, 15.2; 19 F NMR (376MHz, CDCl 3 )δ-75.2(s,3F).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com