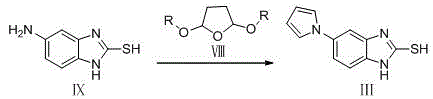
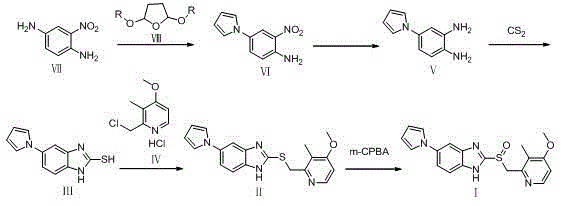
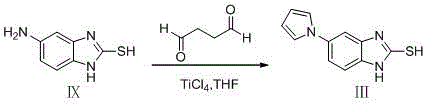
Preparation method of ilaprazole
A technology of ilaprazole and mercaptobenzimidazole, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1 Preparation of 2-nitro-4-(1H-pyrrol-1-yl)aniline (formula VI):
[0067] 120 g (0.784 mol) of 2-nitro-1,4-phenylenediamine (Formula VII), add 12 mL of glacial acetic acid, 1200 mL of water, and 1200 mL of dichloromethane, raise the temperature to reflux, and dissolve all the raw materials, add 2,5 - 232.8 mL (1.960 mol) of dimethoxytetrahydrofuran, refluxed for 6 h, and TLC monitored the completion of the reaction. The reaction liquid was cooled, and the pH was adjusted to 7-8 with 5% sodium hydroxide solution. A large amount of solids were precipitated, and the filtrate was separated by suction filtration. The dichloromethane layer was washed with saturated brine, and dried over sodium sulfate. The aqueous layer was extracted with 1200 mL of dichloromethane. Add 1000 mL of dichloromethane to the filter cake, stir, and filter with suction. The organic phases were combined, washed with saturated brine, and dried over sodium sulfate. The desiccant was filtered...
Embodiment 2
[0068] Example 2 Preparation of 4-(1H-pyrrol-1-yl)-1,2-phenylenediamine (Formula V):
[0069] 2-nitro-4-(1H-pyrrol-1-yl)aniline (formula VI) 120.0 g (0.591 mol) and SnCl 2 2H 2 O 348 g (1.182 mol) was added to 1200 mL of ethanol, heated to reflux for 1 h, and the reaction was complete as monitored by TLC. Ethanol was recovered by distillation under reduced pressure, 1200 mL of water was added to the residue, the pH was adjusted to 9 with 5% NaOH under stirring, 1200 mL of dichloromethane was added, stirred, suction filtered, separated, the aqueous layer was extracted once with dichloromethane, The organic layers were combined, washed with saturated brine, and dried over magnesium sulfate. The desiccant was filtered off and concentrated to obtain 93.2 g of 4-(1H-pyrrol-1-yl)-1,2-phenylenediamine (Formula V), yield: 91.1%. Mp: 75.2-76.1°C; 1 H NMR (DMSO-d 6 , 300MHz): 6.97-6.96(t, 2H), 6.75-6.72(m, 3H), 6.30-6.27(t, 2H), 3.43(s, 4H); ESI-MS m / z: 174.1 [M+H ] + .
Embodiment 3
[0070] Example 3 Preparation of 4-(1H-pyrrol-1-yl)-1,2-phenylenediamine (Formula V):
[0071] 2-Nitro-4-(1H-pyrrol-1-yl)aniline (Formula VI) 10.0 g (0.049 mol), sodium hydrosulfite 25.5 g (0.147 mol), add 100 mL of ethanol and 100 ml of water, heat to reflux 4 h, TLC monitors that the reaction is not complete, add 8.5 g (0.049 mol) of hydrosulfite, reflux for 2 h, and TLC monitors that the reaction is complete. Ethanol was distilled off under reduced pressure, and the aqueous layer was extracted three times with 100 mL of dichloromethane, combined, and dried over magnesium sulfate. The desiccant was filtered off and concentrated to obtain 7.1 g of 4-(1H-pyrrol-1-yl)-1,2-phenylenediamine (Formula V), yield: 84.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com