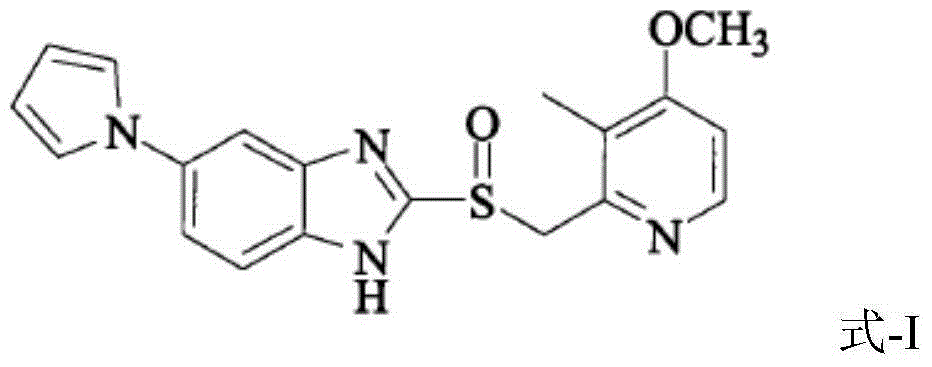
Ilaprazole freeze-dried composition
A technology of ilaprazole and a composition, applied to a freeze-dried composition of ilaprazole and the field of preparation thereof, can solve the problems of poor stability of ilaprazole and the like, and achieve low moisture content and improved compliance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] prescription:
[0041]
[0042] Take 1.3L of water for injection and 0.2L of tert-butanol, add mannitol and edetate disodium under stirring, stir and dissolve, adjust the pH to 9.5-10 with NaOH solution, and add Aipla after the solution temperature drops to about 4°C Stir to dissolve azole, adjust the pH to 9.5-10 with 1mol / L NaOH solution and / or 1mol / L hydrochloric acid solution as needed, add water for injection until the volume of the solvent is 2L. The liquid medicine was sterilized and filtered in turn, and then filled into vials with 2 mL of liquid medicine in each bottle, half-plugged with a butyl rubber stopper.
[0043] Freeze-dry the filled medicinal liquid product. Pre-freezing stage: the product is cooled to -30°C and kept for 2 hours; when the product is heated to 5°C, kept for 2 hours; the product is cooled to -15°C and kept for 2 hours; the product is cooled to -40°C and kept for 2 hours. Sublimation and drying stage: reduce the temperature of the co...
Embodiment 2
[0046] prescription:
[0047]
[0048] Take 1.3L of water for injection and 0.1L of tert-butanol, add mannitol and edetate disodium under stirring, stir and dissolve, adjust the pH to 9.5-10 with NaOH solution, and add Aipla after the solution temperature drops to about 10°C Stir to dissolve azole, adjust the pH to 9.5-10 with 1mol / L NaOH solution and / or 1mol / L hydrochloric acid solution as needed, add water for injection until the volume of the solvent is 2L. The liquid medicine was sterilized and filtered in turn, and then filled into vials with 2 mL of liquid medicine in each bottle, half-plugged with a butyl rubber stopper.
[0049] Freeze-dry the filled medicinal liquid product. Pre-freezing stage: the product is cooled to -30°C and kept for 2 hours; when the product is heated to 5°C, kept for 2 hours; the product is cooled to -15°C and kept for 2 hours; the product is cooled to -40°C and kept for 2 hours. Sublimation and drying stage: reduce the temperature of the c...
Embodiment 3
[0052] prescription:
[0053]
[0054] Take 1.5L of water for injection and 0.15L of tert-butanol, add mannitol and edetate disodium under stirring, after stirring and dissolving, adjust the pH to 9.5-10 with NaOH solution, and add Aipla after the temperature of the solution drops to about 6°C Stir to dissolve azole, adjust the pH to 9.5-10 with 1mol / L NaOH solution and / or 1mol / L hydrochloric acid solution as needed, add water for injection until the volume of the solvent is 2L. The liquid medicine was sterilized and filtered in turn, and then filled into vials with 2 mL of liquid medicine in each bottle, half-plugged with a butyl rubber stopper.
[0055] Freeze-dry the filled medicinal liquid product. Pre-freezing stage: the product is cooled to -30°C and kept for 2 hours; when the product is heated to 5°C, kept for 2 hours; the product is cooled to -15°C and kept for 2 hours; the product is cooled to -40°C and kept for 2 hours. Sublimation and drying stage: reduce the t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com