Powder injection for treating peptic ulcers and preparation method thereof
A technology for peptic ulcer and powder injection, applied in the direction of digestive system, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 : the formula selection of ilaprazole sodium freeze-dried powder injection of the present invention
[0046] 1. Selection of excipients
[0047] Experiments have shown that when the mass fraction of the excipient exceeds 30 parts, the excipient cannot be completely dissolved in the solution containing ilaprazole sodium; when the mass fraction of the excipient is less than 10 parts, the ilaprazole Sodium azole is relatively unstable in the solution and is prone to precipitation; when the mass fraction of the excipient exceeds 18 parts, mannitol will precipitate out after the solution is left for several hours.
[0048] In the aqueous solution containing one part of ilaprazole sodium, add 0, 3, 6, 9, 12, 15, 18 parts of mannitol and 0, 0.1, 0.2, 0.3 parts of edetate disodium respectively, and combine according to the arrangement According to the stability of each prescription under dark conditions, it was determined that when 18 parts of mannitol and 0.3 part...
Embodiment 2
[0057] Example 2 : Preparation and detection of ilaprazole sodium freeze-dried powder injection
[0058] 1. Preparation of ilaprazole sodium freeze-dried powder injection
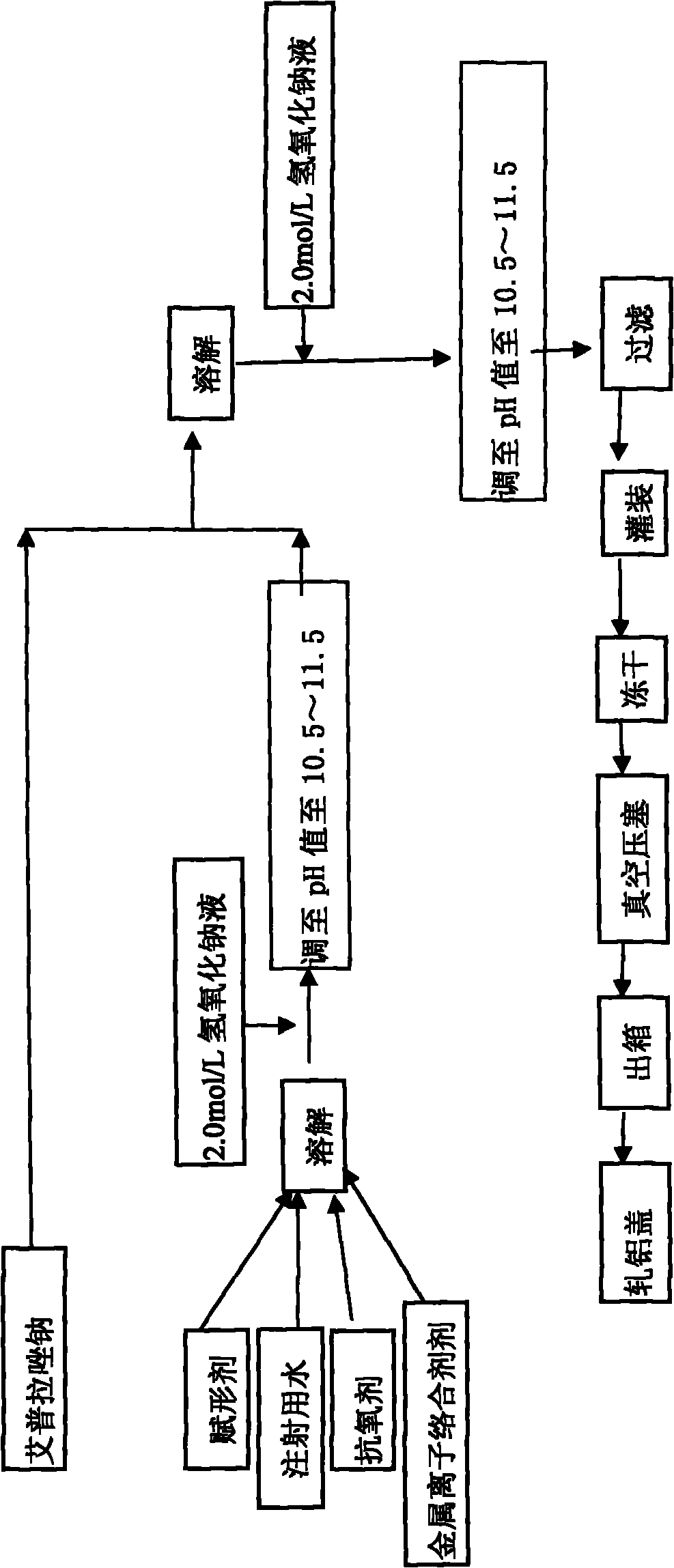
[0059] refer to figure 1 As shown in the process flow diagram, 1000 bottles of ilaprazole sodium freeze-dried powder injection (marked amount: containing 10g ilaprazole sodium) are prepared by the method of the present invention, and the specific process comprises the following steps:
[0060] 1) Take materials according to the following weight: mannitol 180.0g, sodium thiosulfate 4.0g, edetate disodium 2.0g, ilaprazole sodium 11.0g;
[0061] 2) Dissolve mannitol, sodium thiosulfate, and edetate disodium in 1300g water for injection at 4°C, adjust the pH value to 10.5 with 2mol / L sodium hydroxide solution, add ilaprazole sodium, dissolve fully and then Use 2mol / L sodium hydroxide solution to adjust the pH value to 10.5, and finally add 4°C water for injection to 1500g;
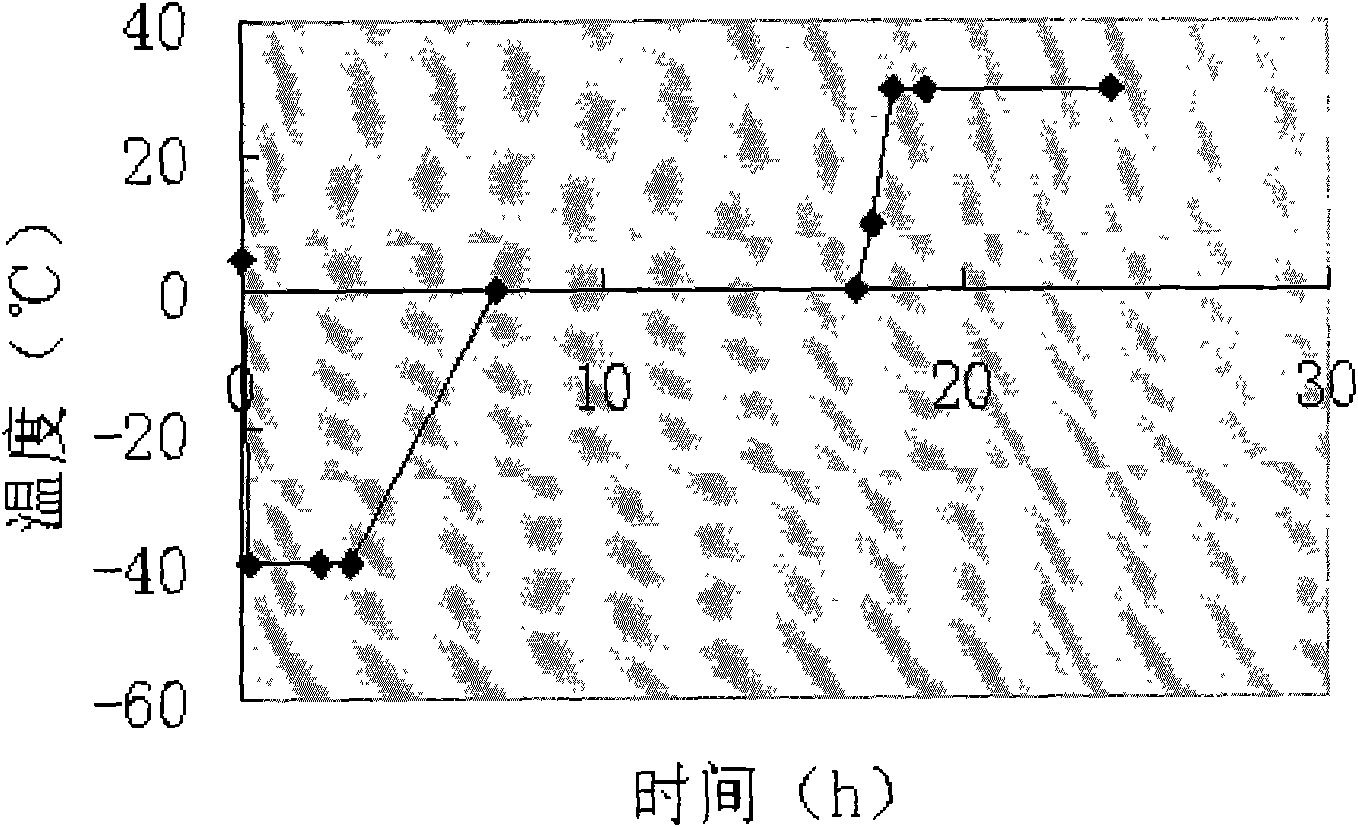
[0062] 3) Sterilize by filterin...
Embodiment 3
[0070] Example 3 : Preparation and detection of ilaprazole sodium freeze-dried powder injection
[0071] This embodiment is to adopt the method of the present invention to prepare 1000 bottles of ilaprazole sodium freeze-dried powder injection (marked amount: containing 10g ilaprazole sodium), and the specific process includes the following steps:
[0072] 1) Take materials according to the following weight: xylitol 150.0g, ilaprazole sodium 11.0g;
[0073] 2) Dissolve xylitol with 1200g, 10°C water for injection, adjust the pH value with 2mol / L sodium hydroxide solution, add ilaprazole sodium, fully dissolve and then adjust with 2mol / L sodium hydroxide solution pH value to 11.5, finally add water for injection at 10°C to 1500g;
[0074] 3) Sterilize by filtering 3 times with a filter membrane with a pore size of 0.2 μm;
[0075] 4) Use an automatic filling machine (Nanjing Bojian Technology Co., Ltd. KFG~300 linear semi-dropper aseptic filling machine) to dispense the fil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com